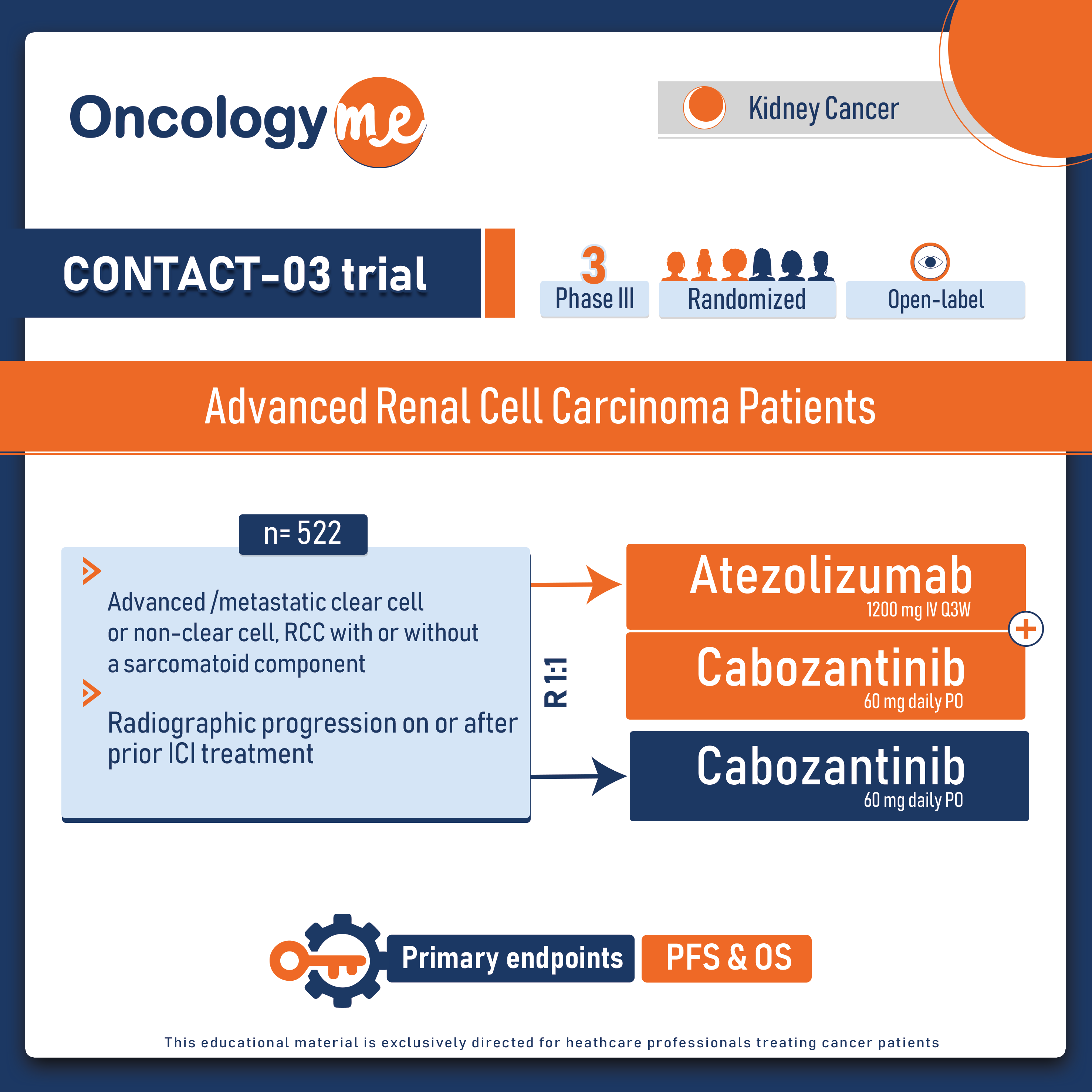
.In the open-label, phase III CONTACT-03 trial, The addition of atezolizumab to cabozantinib did not improve clinical outcomes in patients with locally advanced or metastatic clear cell or non-clear cell (papillary or unclassified only) renal cell carcinoma (RCC) who progressed during or after immune checkpoint inhibitor therapy (either combination or monotherapy) and led to increased toxicity.
In this multicentre, randomised trial, carried out in 15 countries in Asia, Europe, North America, and South America. 522 eligible patients with locally advanced or metastatic renal cell carcinoma whose disease had progressed with immune checkpoint inhibitors were randomly assigned (1:1) to receive atezolizumab (1200 mg intravenously every 3 weeks) plus cabozantinib (60 mg orally once daily) or cabozantinib alone. The two primary endpoints were progression-free survival (PFS) and overall survival (OS).
After median follow-up was 15·2 months, Median PFS was 10·6 months with atezolizumab–cabozantinib vs 10·8 months with cabozantinib (HR=1·03, p=0·78). Median OS was 25·7 months with atezolizumab–cabozantinib and was not evaluable with cabozantinib (HR=0·94, p=0·69). Notably, toxicity was higher with the combination of cabozantinib and atezolizumab, Serious adverse events occurred in 48% of patients receiving atezolizumab–cabozantinib and 33% of patients treated with cabozantinib; adverse events leading to death occurred in 6% patients in the atezolizumab–cabozantinib group and 4% in the cabozantinib group.
These results should discourage sequential use of immune checkpoint inhibitors in patients with renal cell carcinoma.
.png)