FDA updates/approvals
"AHOD1331 study - Bv-AVEPC approved for 2-year-olds and older with previously untreated high risk cHL "
Mar 26, 2023

On November 10, 2022, the FDA approved #brentuximab vedotin in combination with doxorubicin, vincristine, etoposide, prednisone, and cyclophosphamide for pediatric...
After a median follow-up of 67.6 months, the 5-year EFS rate was 70.7% in the carboplatin arm vs 64.1% in the control arm (HR, 0.798; P = 0.081). In patients younger than 50 years, the 5-year EFS rate was 74.2% vs 61.7%, respectively (HR, 0.642; P = .004).
Oncologyme
/ Apr 30, 2023

On December 9, 2022, #FDA has approved #Atezolizumab for adult and pediatric unresectable or metastatic #alveolar soft part #sarcoma (ASPS). The...
Cemiplimab FDA approval in NSCLC
Oncologyme
/ Mar 26, 2023
.png)
On November 8, 2022, the FDA approved cemiplimab-rwlc in combination with platinum-based chemotherapy as first line therapy for patients with...
Dabrafenib + Trametinib FDA approval for treatment of adult and pediatric patients (aged 6 and older) with unresectable or metastatic solid tumors with BRAF V600E mutation
Oncologyme
/ Mar 26, 2023

On June 22, 2022, the #FDA granted an accelerated approval to #dabrafenib in combination with #trametinib for treatment of adult...
FDA approval of bevacizumab biosimilar
Oncologyme
/ Feb 15, 2023

On September 28th 2022, the FDA has approved Celltrion’s #bevacizumab biosimilar (bevacizumab-adcd) in six types of cancers which are: metastatic...
FDA approval of tremelimumab in combination with durvalumab in metastatic NSCLC
Oncologyme
/ Mar 26, 2023

On November 10, 2022, the FDA approved #tremelimumab in combination with #durvalumab and platinum-based chemotherapy as first line therapy for...
FDA Approves Olaparib Combined with Abiraterone and Prednisone as First-line Treatment for BRCA-Mutated Metastatic Castration-Resistant Prostate Cancer
Oncologyme
/ Sep 04, 2023

FDA Grants Regular Approval to Pralsetinib for Adult Patients with Metastatic RET Fusion-Positive NSCLC
Oncologyme
/ Oct 04, 2023

FDA safety recommendation
Oncologyme
/ Mar 26, 2023
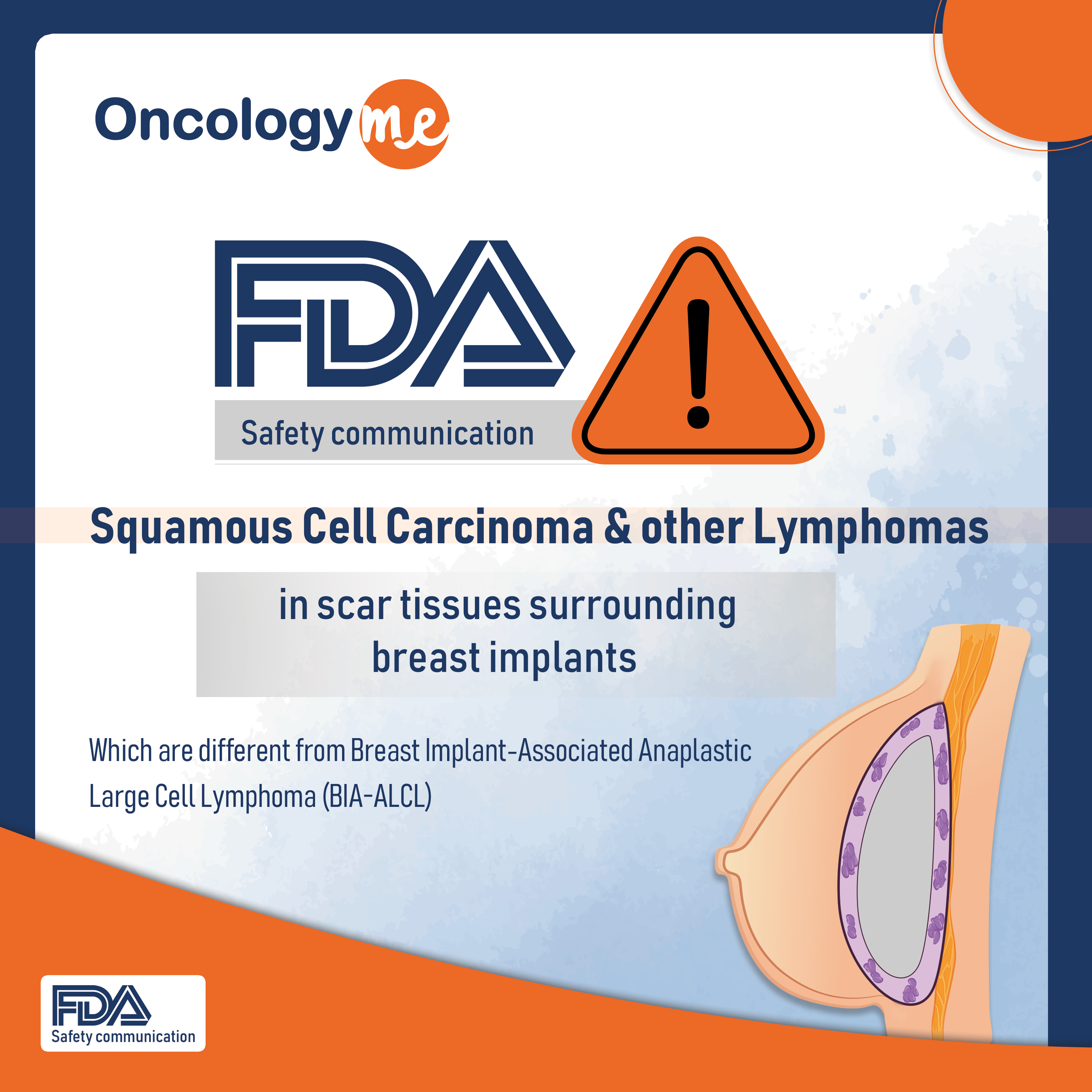
On Sept 8, 2022, the #FDA issued a safety communication to highlight reports of squamous cell carcinoma and various #lymphomas...
HIMALAYA trial
Oncologyme
/ Mar 26, 2023
.png)
The #FDA has approved Tremelimumab plus Durvalumab for patients with unresectable #Hepatocellular Carcinoma. This approval was based on the results...
Latest Articles
- 1
- 2
.png)