Immunotherapy
After a median follow-up of 67.6 months, the 5-year EFS rate was 70.7% in the carboplatin arm vs 64.1% in the control arm (HR, 0.798; P = 0.081). In patients younger than...

On December 9, 2022, #FDA has approved #Atezolizumab for adult and pediatric unresectable or metastatic #alveolar soft part #sarcoma (ASPS). The...
CheckMate 914 trial: adjuvant Immunotherapy in Renal Cell Carcinoma

In the phase 3 CheckMate 914 trial, Adjuvant #nivolumab plus #ipilimumab failed to improve the clinical outcomes in patients with...
New FDA approval for Pembrolizumab in the adjuvant treatment of NSCLC.

On January 26, 2023, the #FDA has approved #pembrolizumab for adjuvant treatment of NSCLC patients following complete resection and platinum-based...
PURE 01 trial: Neoadjuvant Pembrolizumab for Muscle-invasive Bladder Carcinoma

In the phase II PURE-01 trial, #neoadjuvant #pembrolizumab has shown to improve the clinical outcomes in patients with muscle-invasive #bladder...
OS: Low dose Nivolumab + Metronomic chemotherapy

In a randomized phase III Indian study, the addition of low dose nivolumab to chemotherapy improved overall survival in patients...
KEYNOTE-042 study - 1st Line Pembrolizumab 5y OS

First-line #pembrolizumab monotherapy continued to show durable superiority over chemotherapy after 5 years of follow-up in #PD-L1–positive, locally advanced/metastatic NSCLC...
CHOICE-01 study - Toripalimab with chemotherapy investigated as 1st line

In the multicentre, randomized CHOICE-01 study, #Toripalimab with chemotherapy was investigated as first-line treatment in treatment-naïve patients with advanced #NSCLC...
COSMIC-313 trial

In COSMIC-313 trial presented by Dr. Toni Choueiri, #cabozantinib (C) in combination with #nivolumab (N) and #ipilimumab (I) improved PFS...
FDA approval of tremelimumab in combination with durvalumab in metastatic NSCLC

On November 10, 2022, the FDA approved #tremelimumab in combination with #durvalumab and platinum-based chemotherapy as first line therapy for...
Assessing the value of systemic Therapy and radiation therapy in Stage I Nodal Marginal Zone Lymphoma

In a large Propensity score matched retrospective study was conducted to assess the value of systemic Therapy and radiation therapy...
Cemiplimab FDA approval in NSCLC
.png)
On November 8, 2022, the FDA approved cemiplimab-rwlc in combination with platinum-based chemotherapy as first line therapy for patients with...
COSMIC-312 trial

In COSMIC-312 trial, first line #Cabozantinib plus #atezolizumab improved PFS versus #sorafenib for patients with advanced HCC. In this phase...
HIMALAYA trial
.png)
The #FDA has approved Tremelimumab plus Durvalumab for patients with unresectable #Hepatocellular Carcinoma. This approval was based on the results...
IPSOS trial

In IPSOS trial Presented by Dr. Siow Ming Lee, 1L #atezolizumab significantly improved OS vs single-agent chemo in platinum-ineligible pts...
SWOG S1801 trial

In SWOG S1801 study presented by Dr. Sapna Patel, #neoadjuvant treatment (NAT) with #pembrolizumab showed a significant EFS benefit compared...
FDA approval of bevacizumab biosimilar

On September 28th 2022, the FDA has approved Celltrion’s #bevacizumab biosimilar (bevacizumab-adcd) in six types of cancers which are: metastatic...







.png)


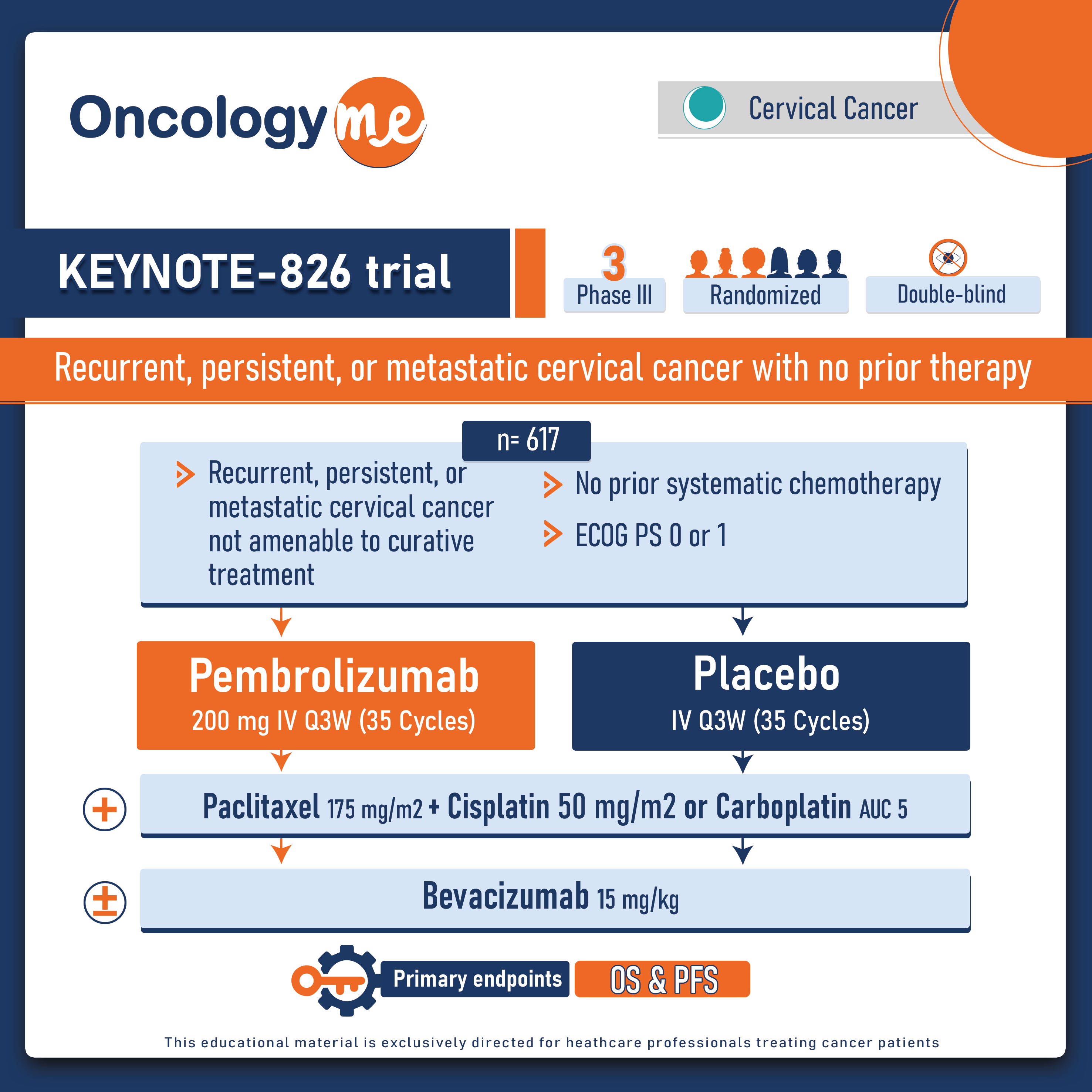
.png)