Sarcoma
After a median follow-up of 67.6 months, the 5-year EFS rate was 70.7% in the carboplatin arm vs 64.1% in the control arm (HR, 0.798; P = 0.081). In patients younger than...

On December 9, 2022, #FDA has approved #Atezolizumab for adult and pediatric unresectable or metastatic #alveolar soft part #sarcoma (ASPS). The...
HYPORT-STS - Moderately Hypofractionated Pre-Operative Radiotherapy
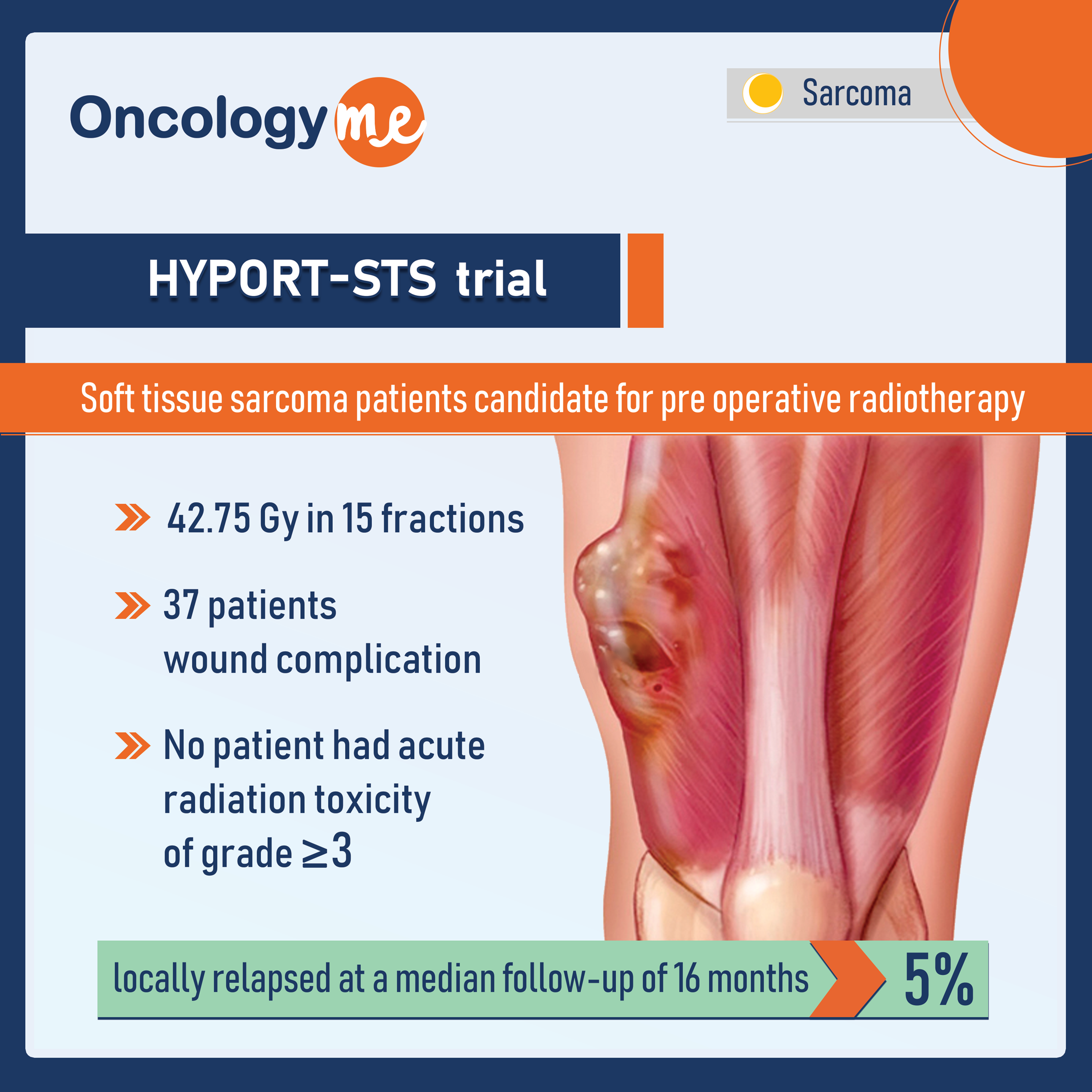
In the phase 2 trial HYPORT-STS conducted at MD Anderson Cancer Center, moderately hypofractionated preoperative radiotherapy to patients with soft...
EURO EWING 2012 trial - Standard U.S. chemotherapy regimen vs standard European regimen
.png)
In the international phase III, EURO EWING 2012 trial, published in the Lancet, standard U.S. chemotherapy regimen improved the clinical...
FDA approval of bevacizumab biosimilar

On September 28th 2022, the FDA has approved Celltrion’s #bevacizumab biosimilar (bevacizumab-adcd) in six types of cancers which are: metastatic...
JCOG0905 trial

In the Japanese JCOG0905 study presented by Dr. Hiroaki Hiraga, adding Ifosfamide (IF) to adjuvant chemotherapy in high grade osteosarcoma...

.png)