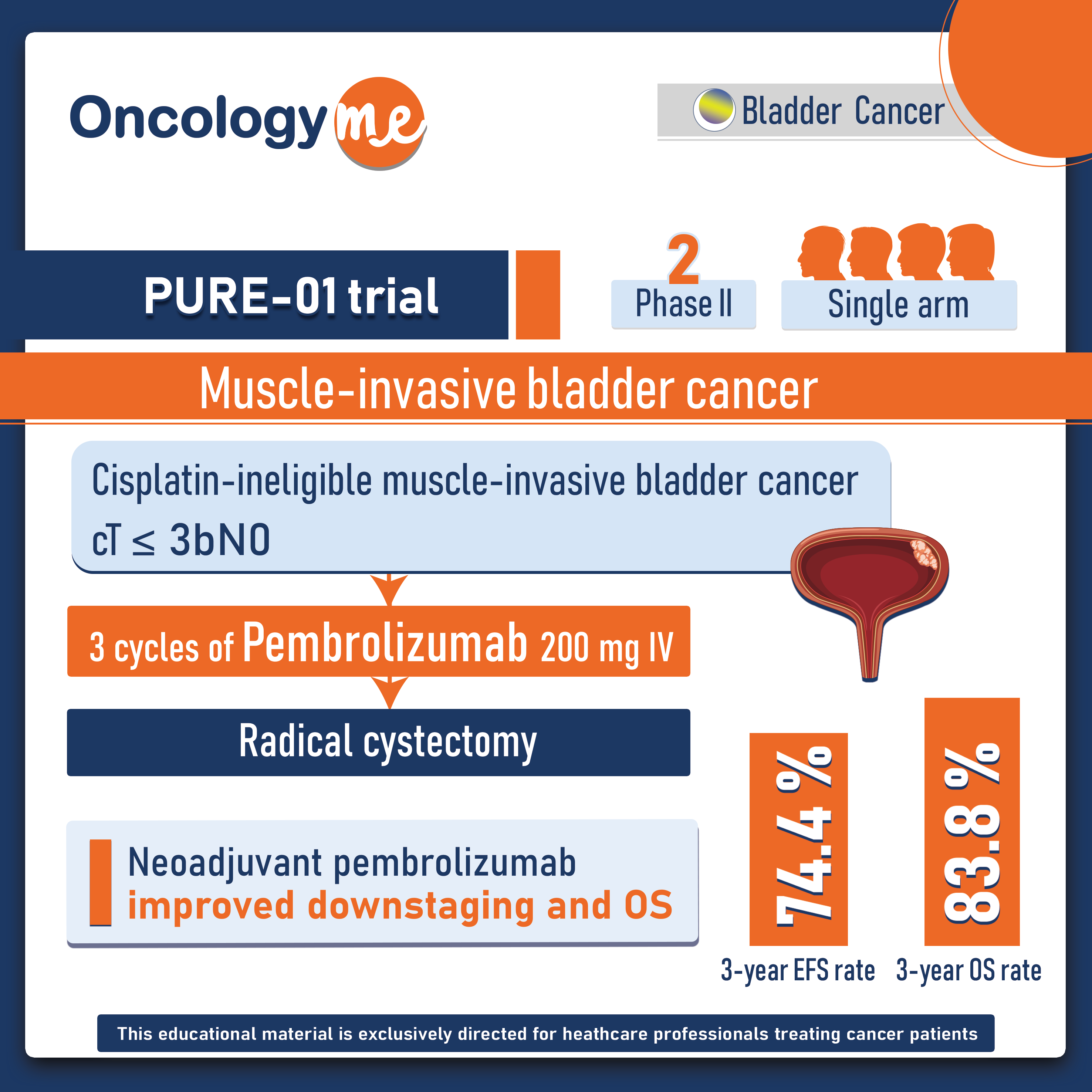
In the phase II PURE-01 trial, #neoadjuvant #pembrolizumab has shown to improve the clinical outcomes in patients with muscle-invasive #bladder cancer (#MIBC).
In this open label, single arm trial, 155 patients with T2-T4a N0 urothelial bladder carcinoma (UBC) with residual disease after transurethral resection of the bladder received 3 cycles of pembrolizumab at the dose of 200mg3 weekly prior to surgery (radical cystectomy).
After median follow up of 39 months, 3-year EFS rate was 74.4% and 3-year OS rate was 83.8% in the ITT population. PD-L1 expression was the strongest predictor of sustained response.
.png)