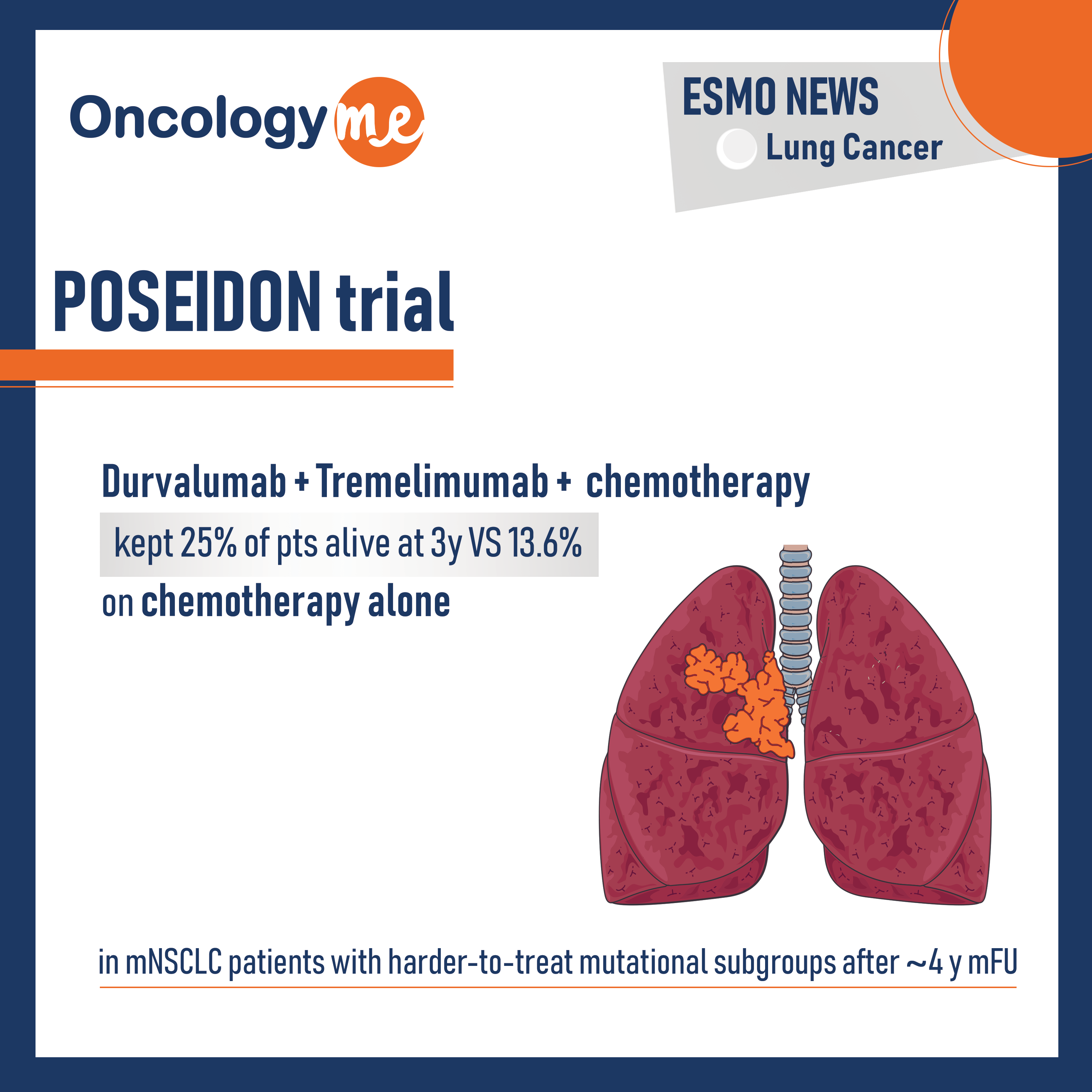
The results of the exploratory analysis from POSEIDON trial were presented by Dr. Melissa Johnson. In this phase III trial, first line Durvalumab (D) + tremelimumab (T) + chemotherapy (CT) continued to show OS benefit vs CT alone (HR 0.75; 95% CI 0.63–0.88) in patients with mNSCLC, with harder-to-treat mutational subgroups such as STK11m, KEAP1m or KRASm. after mFU of ∼4 y, 25.0% of pts alive at 3 y in T+D+CT arm vs 13.6% in CT arm.
.png)