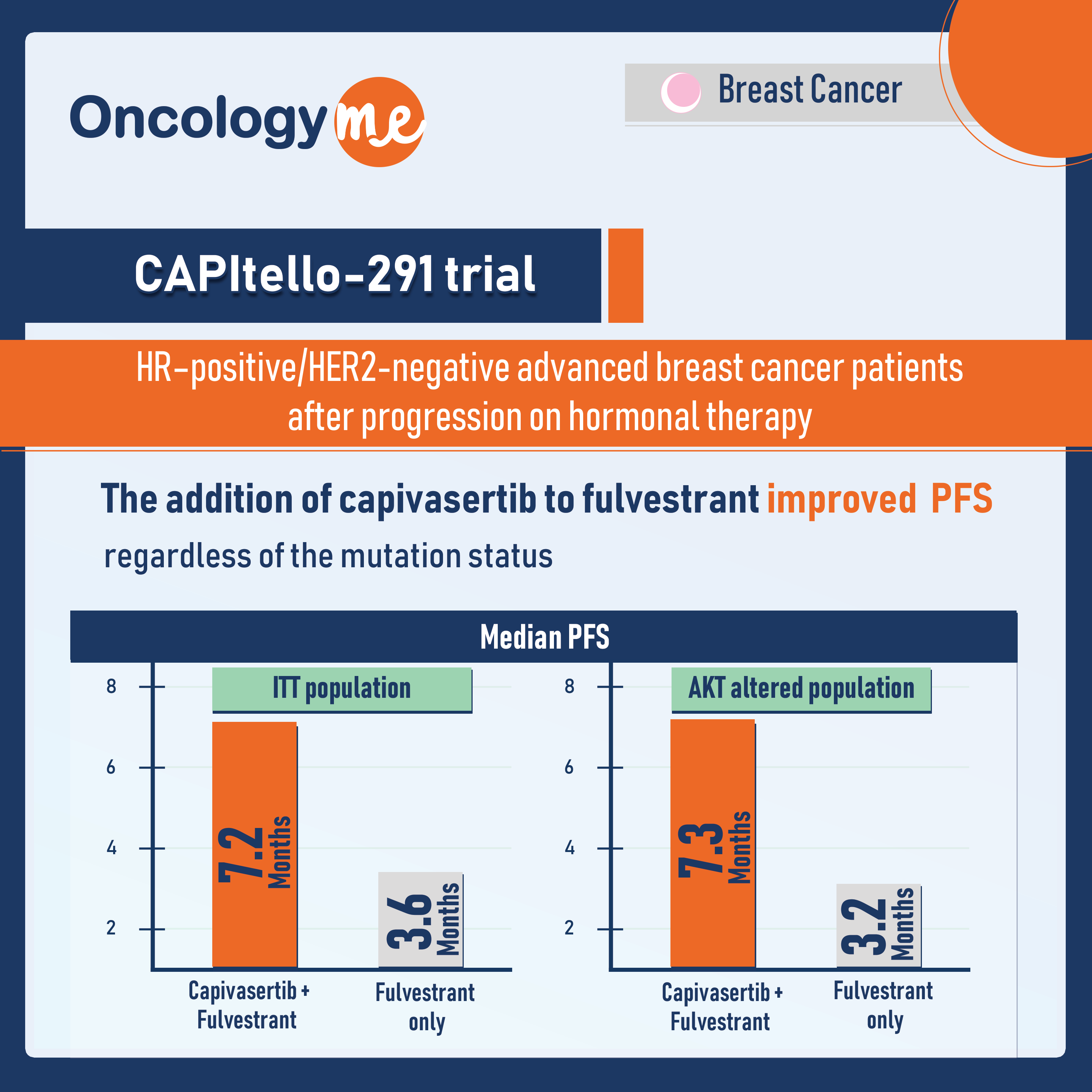
In the phase 3 CAPItello-291 trial, presented at the SABCS, the addition of #capivasertib, an oral AKT inhibitor, to fulvestrant improved PFS in HR–positive/HER2-negative advanced breast cancer patients after progression on hormonal therapy regardless of the mutation status.
In this international, randomized, double-blind phase III trial, Eligible patients that had progressed on AI therapy with or without a CDK4/6 inhibitor were randomized 1:1 to receive fulvestrant with either placebo or capivasertib (400 mg twice daily; 4 days on, 3 days off).
In the ITT population, the median PFS was 7.2 months with capivasertib + fulvestrant and 3.6 months with PBO + fulvestrant (HR= 0.60; p<0.001). In patients with AKT pathway-altered tumors, median PFS was 7.3 months with capivasertib + fulvestrant and 3.1 months with PBO + fulvestrant (HR= 0.50; p<0.001).
.png)