Lung Cancer
New FDA approval for Pembrolizumab in the adjuvant treatment of NSCLC.

On January 26, 2023, the #FDA has approved #pembrolizumab for adjuvant treatment of NSCLC patients following complete resection and platinum-based...
KEYNOTE-042 study - 1st Line Pembrolizumab 5y OS
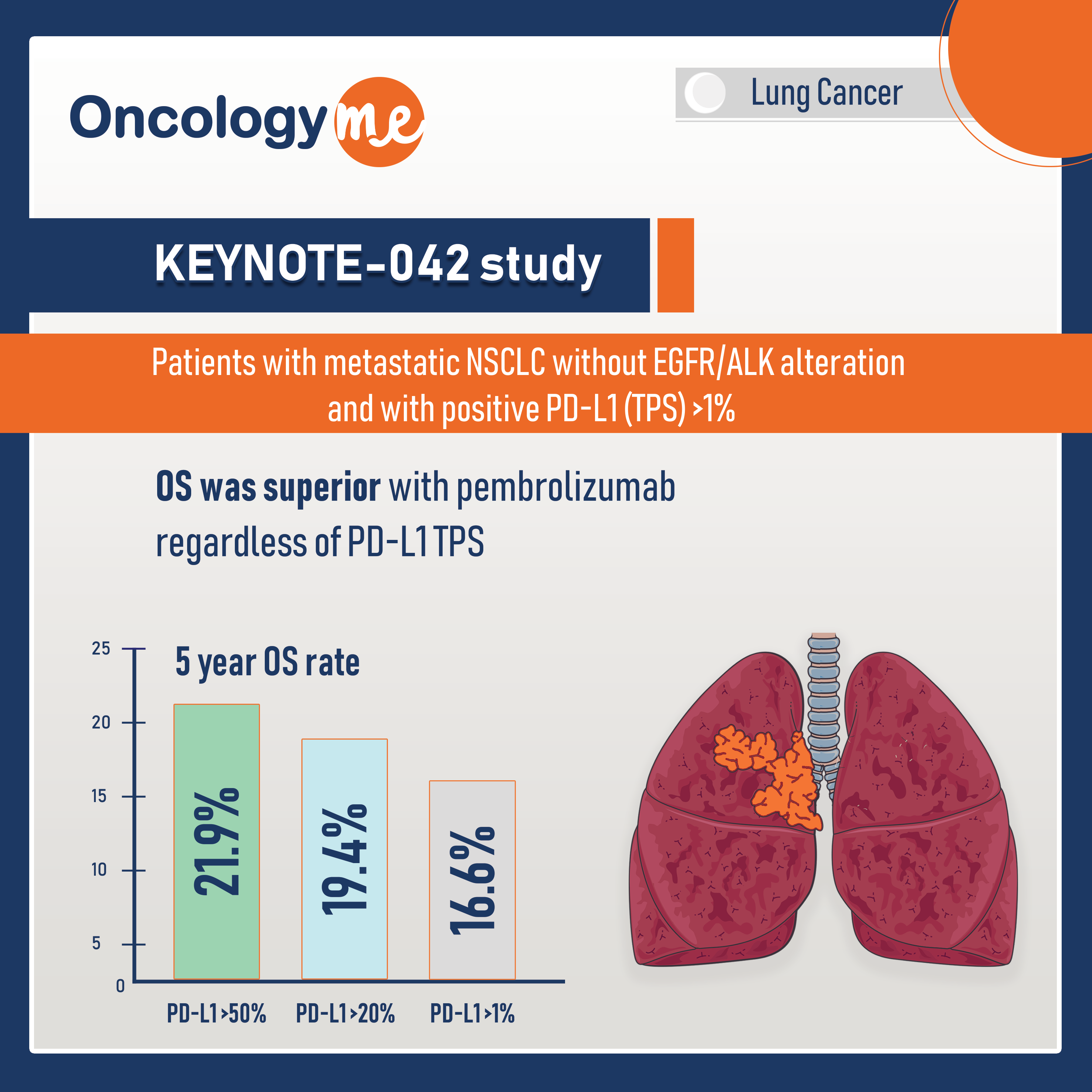
First-line #pembrolizumab monotherapy continued to show durable superiority over chemotherapy after 5 years of follow-up in #PD-L1–positive, locally advanced/metastatic NSCLC...
CHOICE-01 study - Toripalimab with chemotherapy investigated as 1st line
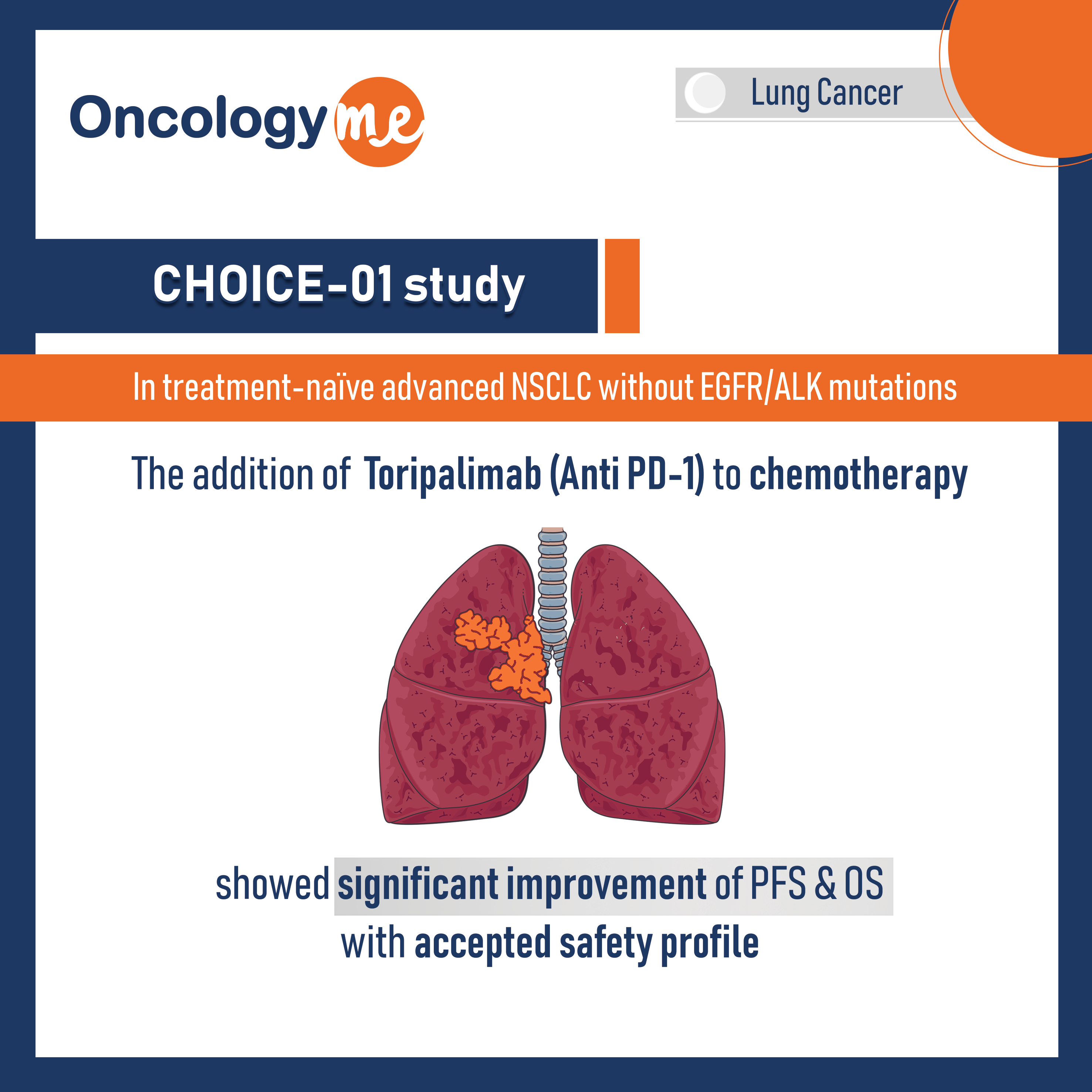
In the multicentre, randomized CHOICE-01 study, #Toripalimab with chemotherapy was investigated as first-line treatment in treatment-naïve patients with advanced #NSCLC...
FDA approval of tremelimumab in combination with durvalumab in metastatic NSCLC

On November 10, 2022, the FDA approved #tremelimumab in combination with #durvalumab and platinum-based chemotherapy as first line therapy for...
Cemiplimab FDA approval in NSCLC
.png)
On November 8, 2022, the FDA approved cemiplimab-rwlc in combination with platinum-based chemotherapy as first line therapy for patients with...
POSEIDON trial
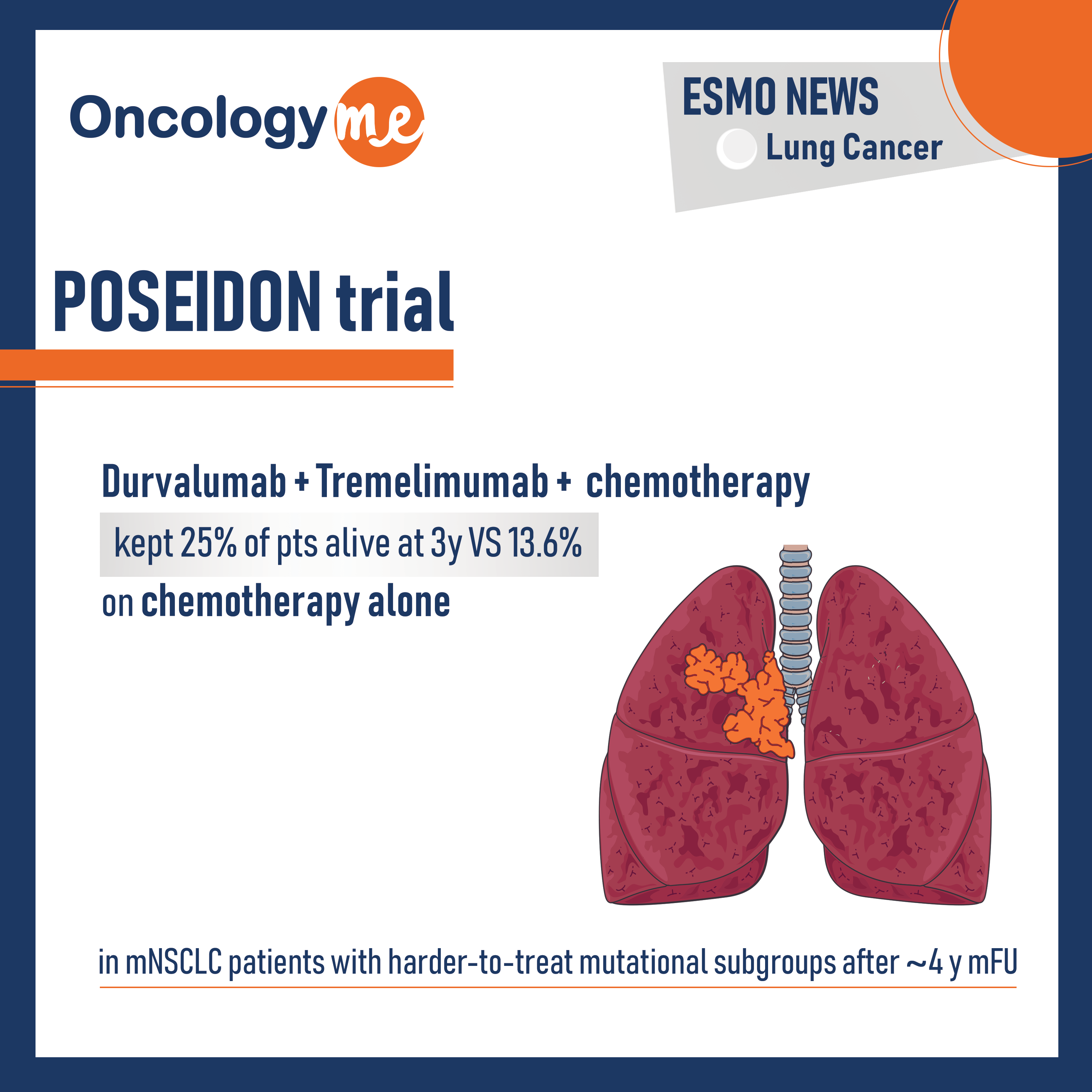
The results of the exploratory analysis from POSEIDON trial were presented by Dr. Melissa Johnson. In this phase III trial,...
DESTINY-Lung02 trial

In DESTINY-Lung02 trial Presented by Dr. Koichi Goto, Trastuzumab deruxtecan (T-DXd) 5.4 mg/kg and 6.4 mg/kg demonstrated clinically meaningful activity...
IPSOS trial

In IPSOS trial Presented by Dr. Siow Ming Lee, 1L #atezolizumab significantly improved OS vs single-agent chemo in platinum-ineligible pts...
CodeBreak 200 trial
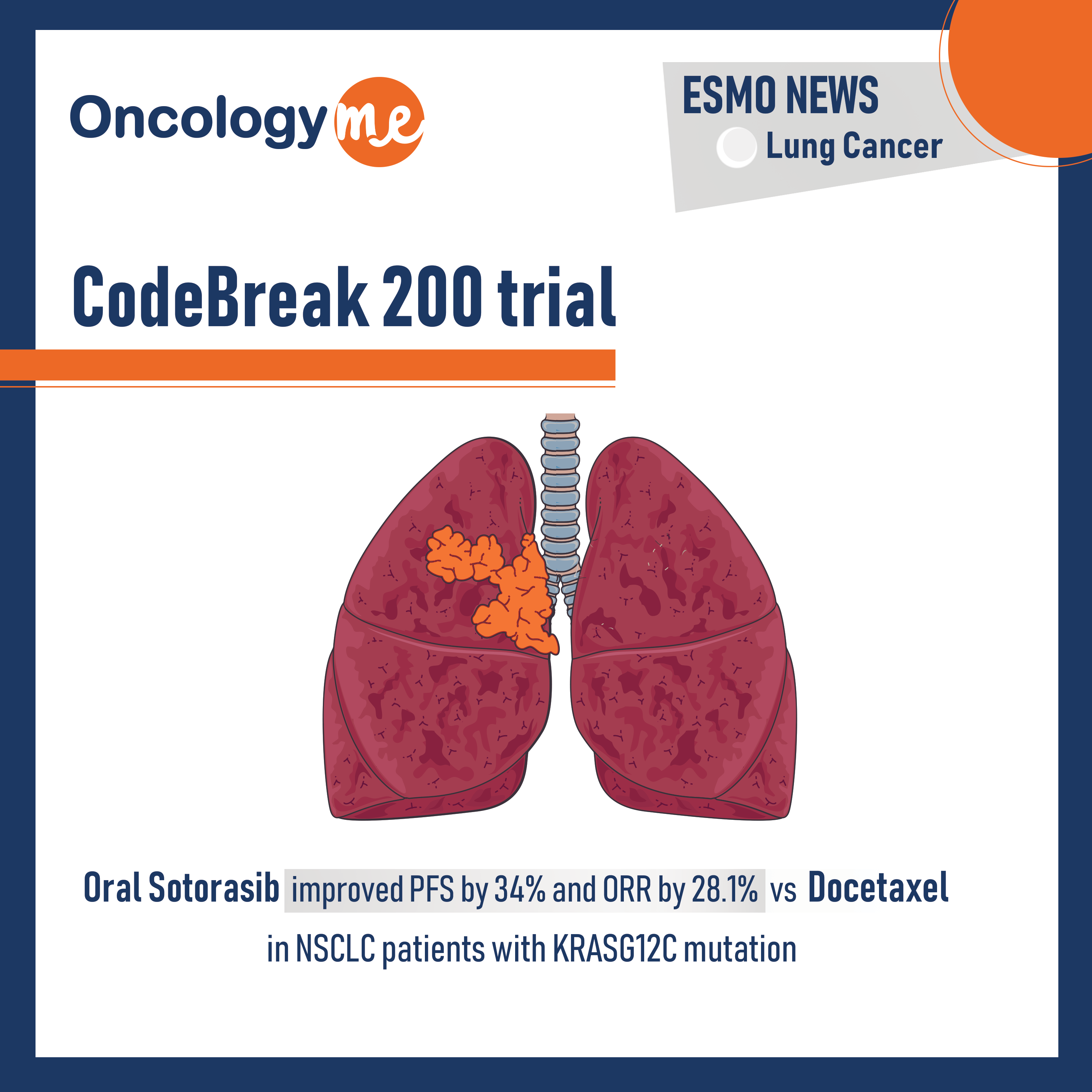
In phase III CodeBreak 200 trial presented by Dr. Melissa Johnson, oral #sotorasib demonstrated superior PFS and ORR compared to...
Trastuzumab deruxtecan FDA approval in metastatic NSCLC

On August 11, 2022, the FDA approved #fam-trastuzumab deruxtecan-nxki (5.4 mg/kg IV every 3 weeks) for adult patients with have...
FDA approval of bevacizumab biosimilar

On September 28th 2022, the FDA has approved Celltrion’s #bevacizumab biosimilar (bevacizumab-adcd) in six types of cancers which are: metastatic...







.png)


.png)