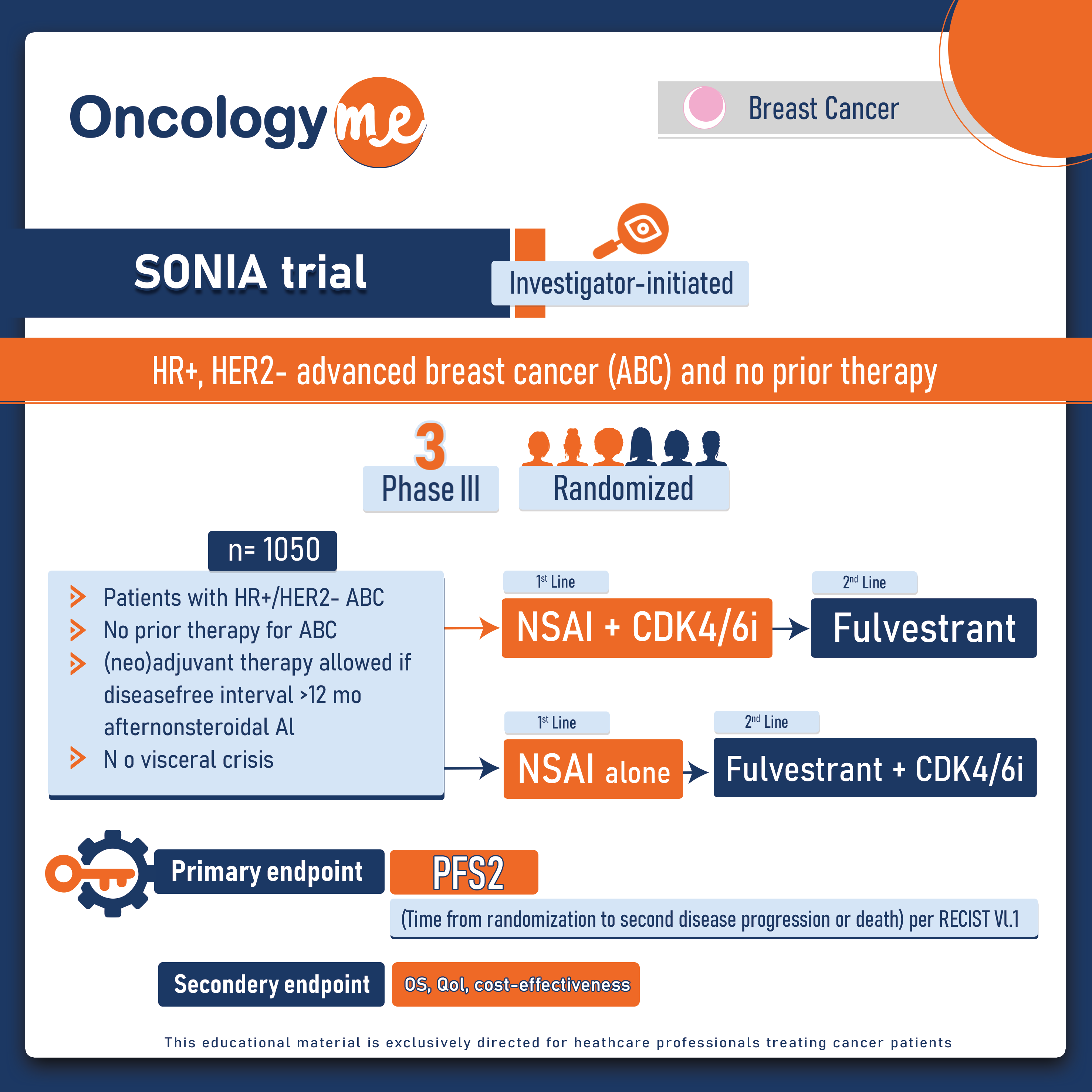
In the phase III SONIA trial, First-line use of CDK4/6 inhibitors in addition to endocrine therapy (ET) wasn’t superior compared to it’s use in the second line setting as regard the progression-free survival and overall survival in women with HR+, HER2- advanced breast cancer (ABC) and no prior therapy.
In this randomized, investigator-initiated, nationwide SONIA trial, Pre- and postmenopausal women with no prior therapy for ABC and measurable disease were enrolled in 74 Dutch hospitals. (Neo)adjuvant therapy was allowed (disease-free interval after non-steroidal aromatase inhibitor (NSAI) >12 months). 1050 eligible patients were randomized 1:1 to receive either first-line therapy with NSAI + CDK4/6i, followed by fulvestrant (F) upon disease progression or first-line NSAI alone, followed by F + CDK4/6i on progression. Choice between one of the available CDK4/6i (abemaciclib, palbociclib, ribociclib) was a stratification factor and left to the discretion of the treating physician. The primary endpoint is time from randomization to second objective disease progression (PFS2). Secondary endpoints include OS, safety, quality of life, and cost-effectiveness.
After a median follow-up of 37.7 months, median PFS2 was 31.0 months with first-line CDK4/6i use and 27.8 months with second-line CDK4/6i use (HR 0.87, P = 0.14). Median OS reached 45.9 months with first-line CDK4/6i use and 53.7 months with second-line CDK4/6i use (HR 0.98, 95% CI [0.80, 1.20]; P = 0.83). Meanwhile, Patients who received a CDK4/6 inhibitor (CDK4/6i) as initial treatment stayed on the medication for an average of 24.6 months, while those who received it as a second-line treatment only stayed on it for 8.1 months - a difference of 16.5 months. Correspondingly, the first-line CDK4/6i strategy resulted in higher rates of grade ≥3 adverse events.
Although SONIA trial provides a new insights to sequencing CDK4/6i in the treatment paradigm and avoids serious financial toxicity, some factors should be put into consideration. Firstly, more than 90% of patients in the study received palbociclib, which is now considered the least attractive CDK4/6i to use in first line because of its lack of OS benefit. Secondly, in the second-line setting, other treatment options are now available, such as alpelisib plus fulvestrant and elacestrant, along with other emerging agents and combinations.
.png)