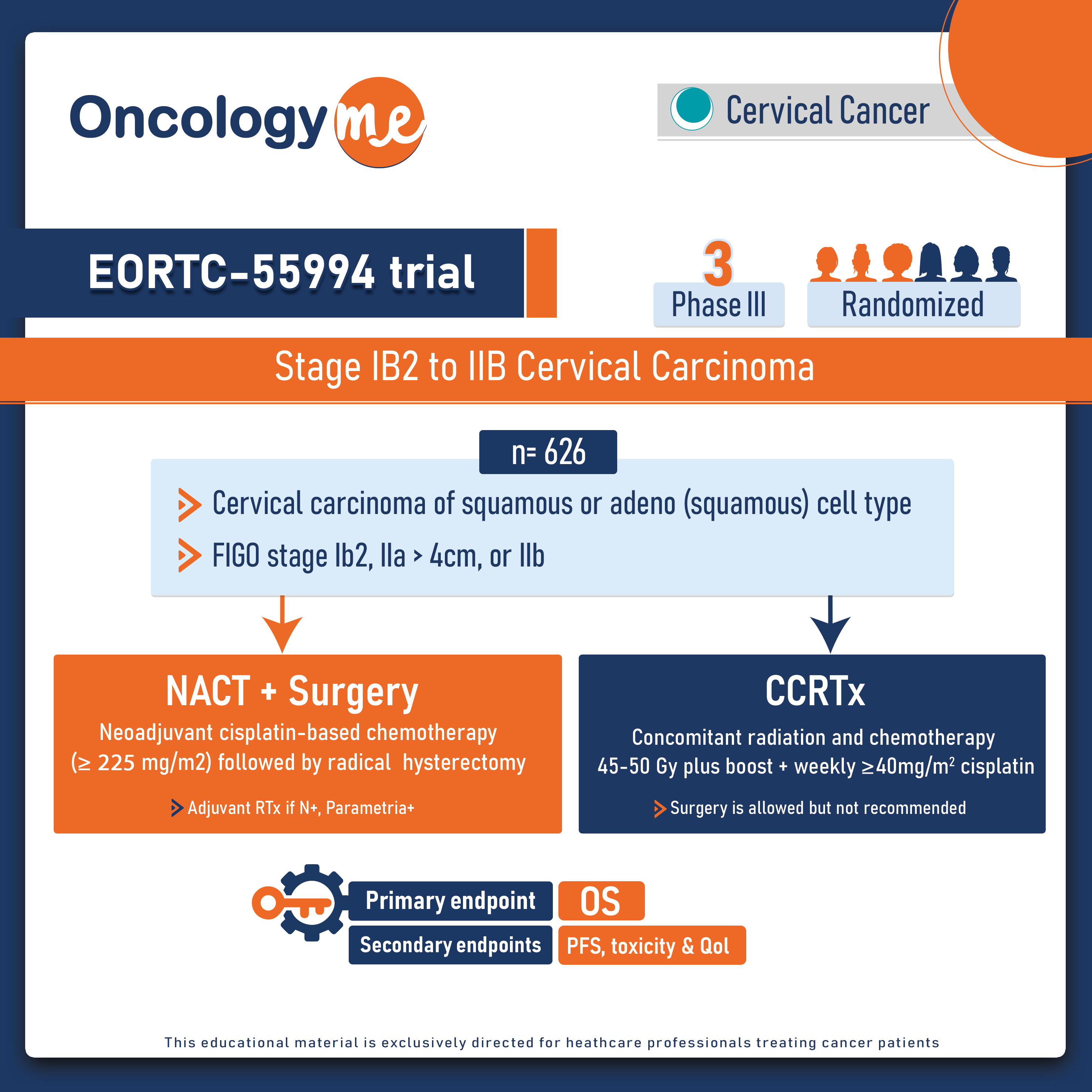
In the multicentre, phase III EORTC-55994 trial, recently published in the Journal of Clinical Oncology, neoadjuvant chemotherapy plus surgery (NACT-S) wasnot superior when compared to concomitant chemoradiotherapy (CCRT) in patients with stage IB2 to IIB #cervical carcinoma.
In this randomized trial, 626 eligible patients with stage IB2-IIb were randomly assigned to receive either neoadjuvant chemotherapy followed by surgery (n = 314) or concomitant chemoradiotherapy (n = 312). NACT-S involved platinum chemotherapy with a minimum cumulative cisplatin dose of 225 mg/m2 and a cisplatin dose equivalent of at least 75 mg/m2 every 3 weeks, with the possibility of combination chemotherapy. CCRT consisted of cisplatin at five or six weekly doses of 40 mg/m2 during radiotherapy, with external-beam radiotherapy given at a total dose of 45 to 50 Gy to the pelvis followed by brachytherapy.
The primary endpoint was 5-year overall survival in the intent-to-treat population. Secondary end points were progression-free survival, OS, toxicity, and health-related quality of life (HRQOL).
After a median follow-up of 8.7 years, The 5-year OS was not significantly different between NACT-S (72%) and CCRT (76%). Median OS was not estimable in both groups (HR for CCRT versus NACT-S = 0.84, P = 0.240).
PFS at 5 years was 57.0% with NACT-S vs 65.6% with CCRT. Median PFS was 12.2 months with NACT-S vs not estimable with CCRT (HR for CCRT vs NACT-S = 0.72, P = 0.010). Short-term grade ≥ 3 adverse events occurred in 41% of the group given NACT-S vs 23% of the group given CCRT. Long-term grade ≥ 3 adverse events occurred in 21% of patients given CCRT vs 15% of those given NACT-S. Health-related quality of life measured by the EORTC QLC-C30 showed no relevant differences between groups.
This trial is noteworthy due to its multinational cohort and extensive follow-up period, providing robust evidence regarding the efficacy and safety of NACT-S compared to CCRT in this patient population.
.png)