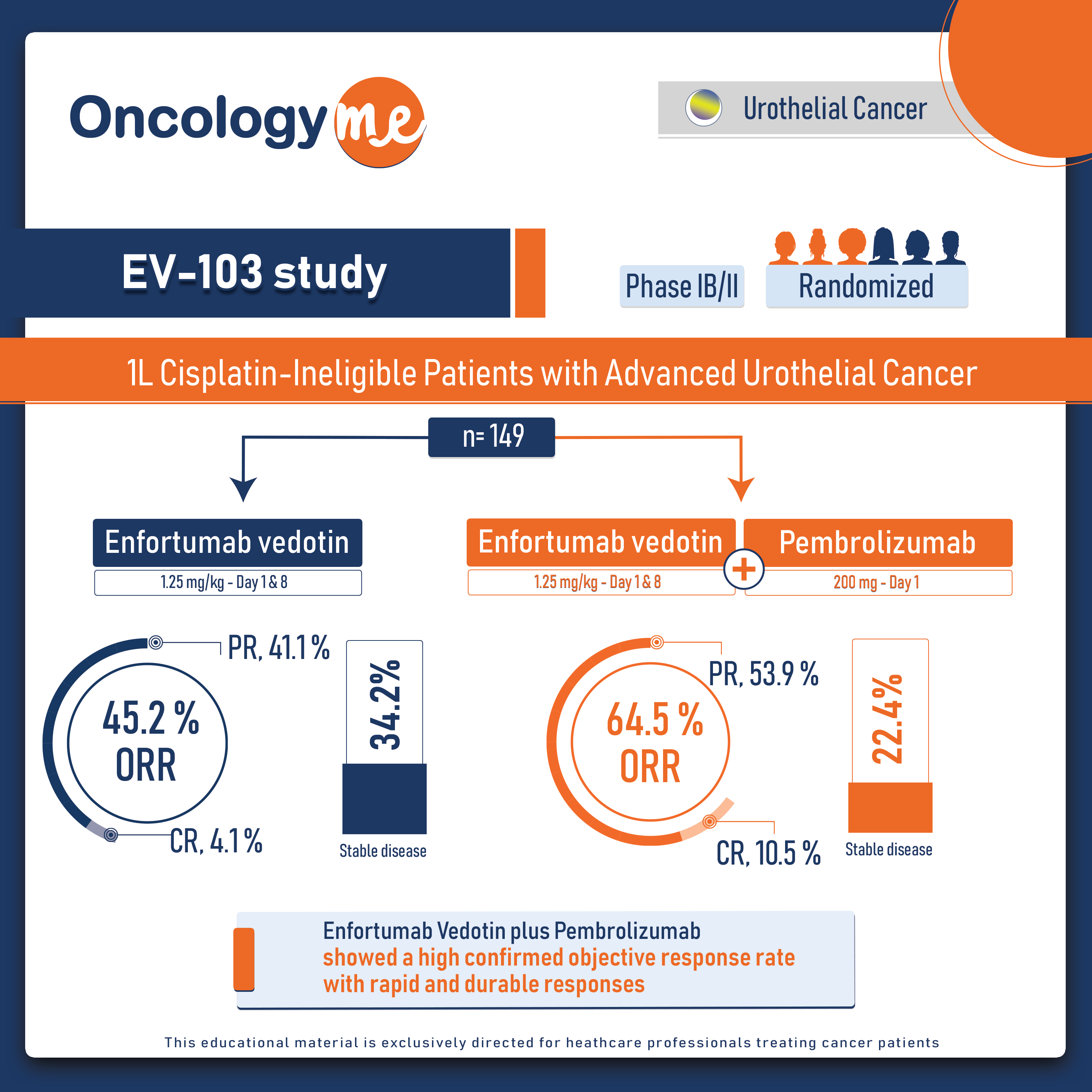
In the phase IB/II EV-103 study, #Enfortumab Vedotin plus #Pembrolizumab showed a high confirmed objective response rate with rapid and durable responses in patients with locally advanced or metastatic #urothelial cancer (la/mUC) who are ineligible for #cisplatin-based therapy , with more than 50% of responders maintaining a response at 12 months.
Enfortumab Vedotin ((EV), an antibody-drug conjugate (ADC), comprises a fully human monoclonal antibody specific for nectin-4 and monomethyl auristatin E (MMAE). EV delivers MMAE to cells expressing nectin-4, leading to cell cycle arrest and cell death. Pembro is an anti–PD-1 antibody that uses the PD-1 receptor as a therapeutic target and has antitumor activity in multiple tumor types.
In Cohort K of the EV-103 study, 149 cisplatin-ineligible patients with previously untreated la/mUC were randomly assigned 1:1 to receive EV (1.25 mg/kg (maximum total dose of 125 mg) as a single IV infusion over 30 minutes on days 1 and 8 of a 3-week cycle) as monotherapy or in combination with Pembro (200 mg as a single intravenous infusion over 30 minutes on day 1 of a 3-week cycle). The primary end point was confirmed objective response rate (cORR). Secondary end points included duration of response (DOR) and safety.
Patients were deemed ineligible for cisplatin-based chemotherapy on the basis of at least one of the following: glomerular filtration rate (GFR) <60 mL/min and ≥30 mL/min, grade ≥ 2 hearing loss, ECOG PS of 2, or Class III heart failure. Patients with an ECOG PS of 2 met additional criteria: HB ≥ 10 g/dL, GFR ≥ 50 mL/min, and no Class III heart failure.
In patients treated with EV + Pembro (N = 76), the cORR by BICR was 64.5%. Eight patients (10.5%) had a complete response; 41 patients (53.9%) achieved a partial response; the median time to response was 2.1 months. A total of 97.1% of assessable patients had a reduction in their target lesions per BICR. The median DOR has not yet been reached; 65.4% of responders maintained a response at 12 months. The DCR was 86.8%. The PFS rate per BICR at 6 and 12 months was 73.8% and 55.1%, respectively, and the OS rate at 6 and 12 months was 88.2% and 80.7%, respectively. The median OS was 22.3m with 54 (of 76) patients remaining on study for OS follow-up at the time of data cutoff.
In the monotherapy arm (N = 73), the cORR per BICR was 45.2%. The DOR was 13.2 months, and the median time to response was 2.1 months. The DCR was 79.5%. The 12-month PFS and OS were 35.8% and 70.7%, respectively.
EV treatment-related adverse events of special interest (AESIs) included skin reactions (EV+Pembro, 67.1%; EV mono, 45.2%), peripheral neuropathy (EV+Pembro, 63.2%; EV mono, 54.8%), and hyperglycemia (EV+Pembro, 14.5%; EV mono, 11.0%). In EV+Pembro, the most frequent (any grade) treatment-emergent adverse events of special interest were severe skin reactions (27.6%), hypothyroidism (13.2%), and pneumonitis (9.2%)
These results provides a strong groundwork for testing this combination in muscle invasive bladder cancer through ongoing phase III studies. "
.png)