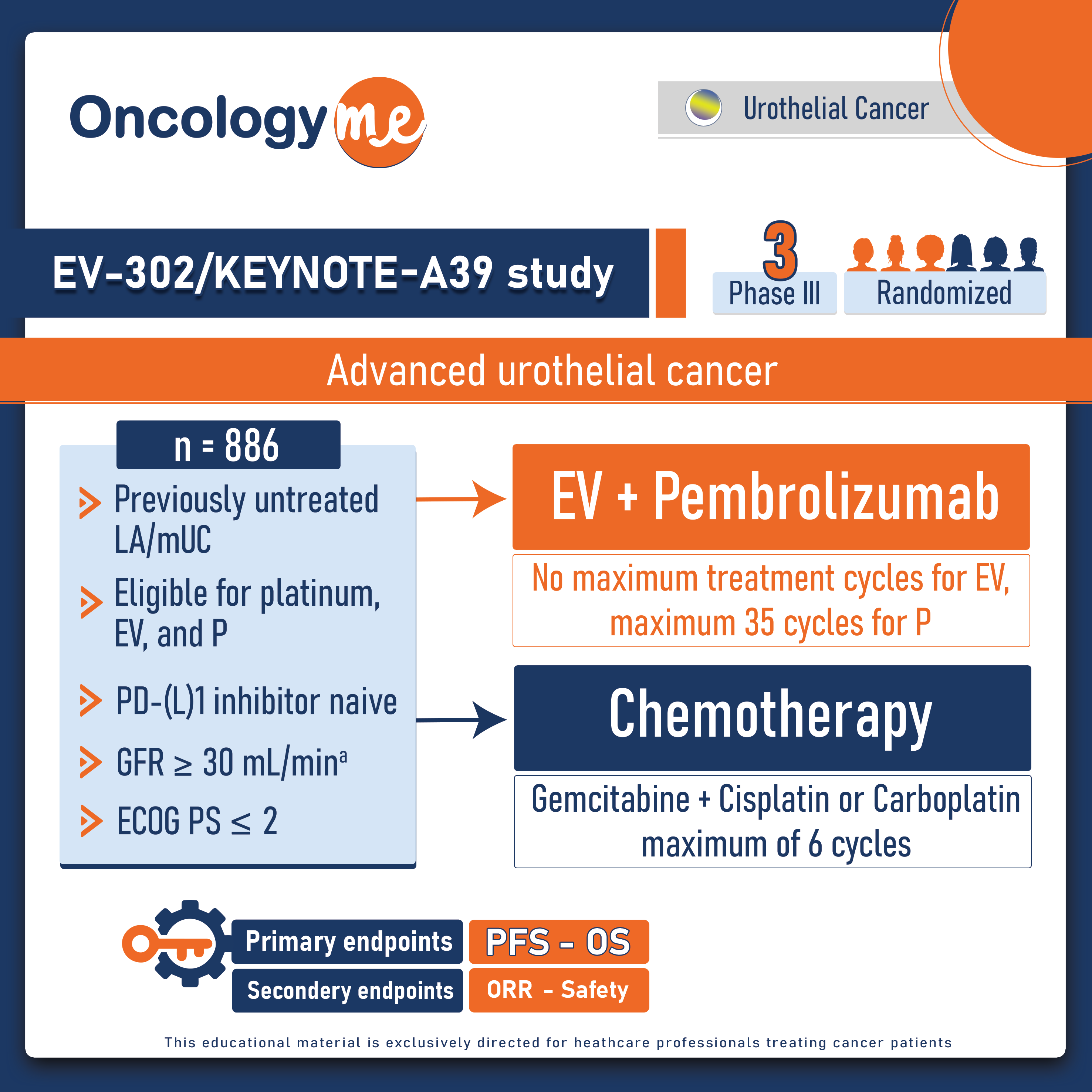
In a groundbreaking development, the combination of #enfortumab vedotin (EV) and #pembrolizumab (P) has set a new precedent in the first line treatment of advanced #urothelial cancer, marking the first significant advancement in care in the past 30 years. This momentous achievement was demonstrated in the open label, randomized phase III EV-302/KEYNOTE-A39 study, presented by Dr. Thomas Powles at #ESMO2023. The study's results revealed a remarkable and significant improvement in overall survival with EV+P compared to chemotherapy.
In this trial, 886 eligible patients with previously untreated locally advanced or metastatic urothelial cancer, regardless of PDL1 expression were randomized in a 1:1 fashion to EV + P (continued until disease progression, clinical progression, unacceptable toxicity, or completion of maximum cycles [35 for pembrolizumab]) versus gemcitabine + cisplatin or carboplatin for a maximum of 6 cycles. Dual primary endpoints were PFS and OS. Select secondary endpoints included overall response rate (ORR) and safety.
Baseline characteristics were well balanced between the 2 arms. 54% of patients were determined to be cisplatin eligible. 22% of patients had evidence of liver metastases. High PD-L1 expression was present in 58% of patients.
After median follow-up of 17.2 months. PFS was significantly prolonged with EV+P vs chemo, reducing the risk of progression or death by 55% (median PFS, 12.5 mo vs 6.3 mo, respectively; HR 0.45, P<0.00001). Subgroup analysis of PFS per BICR demonstrated consistent, clinically meaningful benefits across all evaluable subgroups.
OS was nearly doubled in the EV+P, with median OS of 31.5 and 16.1 months in the EV +P and chemotherapy arms, respectively (HR: 0.47, 95% CI: 0.38 – 0.58, p<0.00001). this translated to 53% reduction in the risk of death. The OS survival benefit was consistent with overall population regardless cisplatin eligibility and PD-L1 expression.
Significant improvement was seen in the ORR was (67.7% vs 44.4% in the EV+P and chemo arms, respectively (P<0.00001)).
In terms of safety, grade ≥3 treatment-related adverse events (TRAES) were observed in a lower percentage of patients in the EV+P group (55.9%) compared to the chemotherapy group (69.5%). The most common grade ≥3 TRAES included maculopapular rash, hyperglycemia, and neutropenia in the EV+P group and anemia (31.4%), neutropenia (30.0%), and thrombocytopenia (19.4%) for chemo. Most common grade ≥3 TRAEs of special interest for EV included skin reactions (15.5%), peripheral neuropathy (6.8%), and hyperglycemia (6.1%). Most common grade ≥3 treatment-emergent AEs of special interest for P included severe skin reactions (11.8%).
the findings from the EV-302/KEYNOTE-A39 study represent a historic milestone in the treatment of advanced bladder cancer. For the first time in three decades, platinum-based chemotherapy has been surpassed in terms of overall survival in patients with previously untreated locally advanced and/or metastatic urothelial carcinoma. This breakthrough treatment approach, involving the combination of EV+P, offers new hope and sets a new standard of care for patients with previously untreated locally advanced/metastatic urothelial carcinoma.
.png)