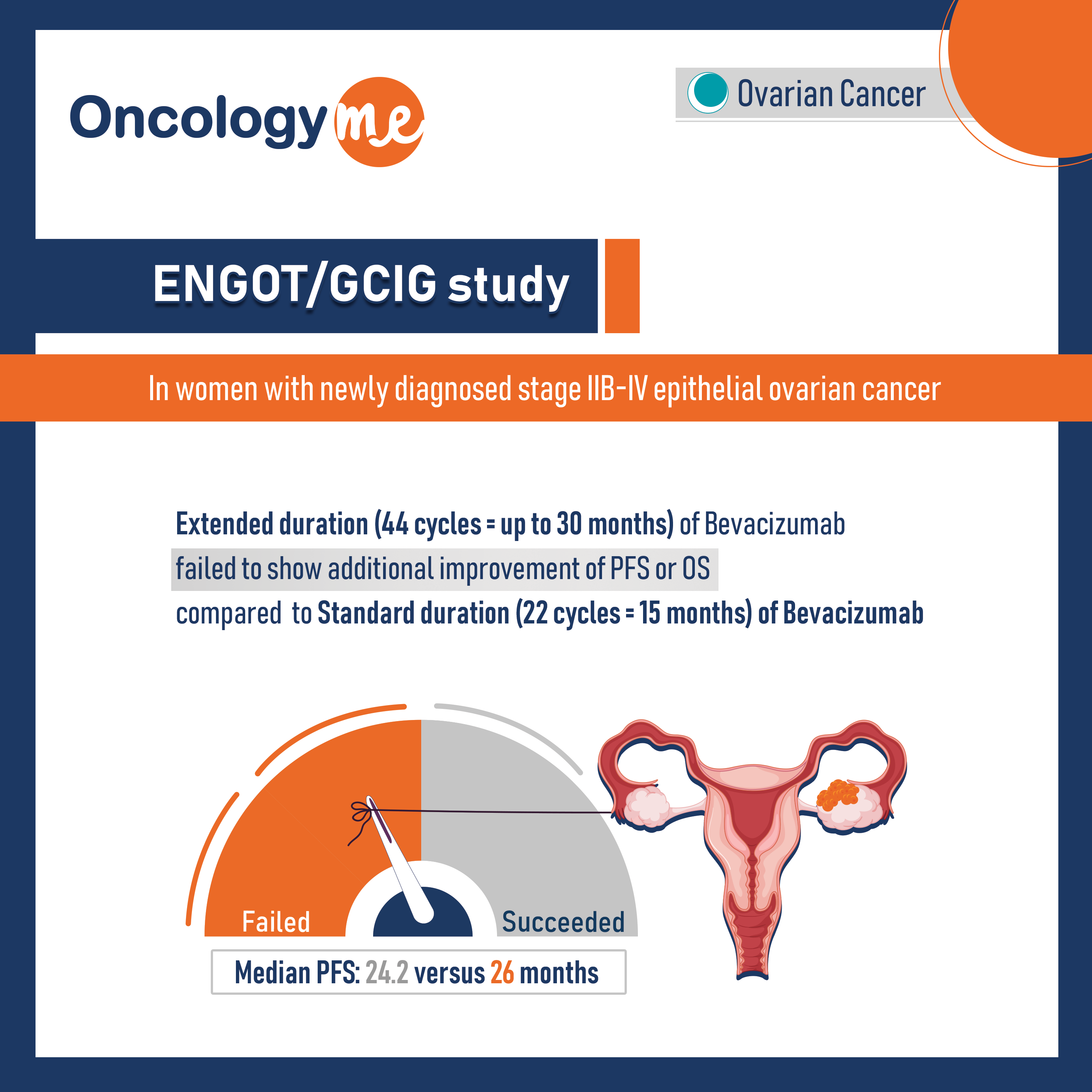
In the prospective, randomized ENGOT/GCIG study by AGO s, GINECO, NSGO study groups, standard duration of #bevacizumab (15 months) treatment was compared to extended duration (up to 30 months) in combination with front-line chemotherapy in women with newly diagnosed stage IIB-IV epithelial #ovarian_cancer.
In this phase III trial, longer duration with bevacizumab failed to show additional improvement of PFS or OS compared to 15 months. Median PFS was 24.2 versus 26.0 months with standard versus extended duration of bevacizumab (HR= 0.99; P = 0.90). Similarly, hazard ratio for OS was 1.04; P = .68.
.png)