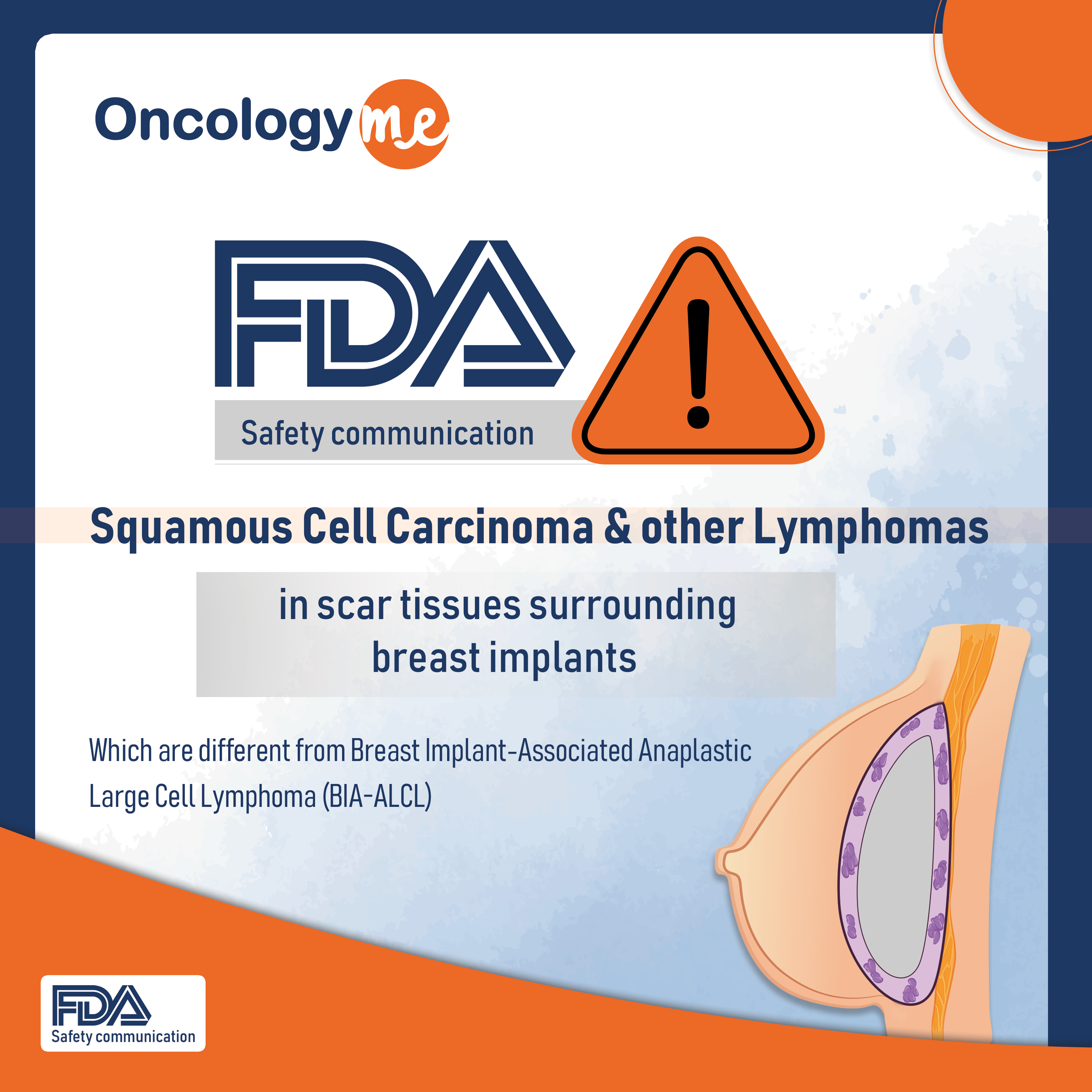
On Sept 8, 2022, the #FDA issued a safety communication to highlight reports of squamous cell carcinoma and various #lymphomas in the scar tissue, or capsule, that surrounds breast #implants which are different from breast implant-associated anaplastic large cell lymphoma (BIA-ALCL), which is also found in the capsule.
A literature review done by the agency, turned up a total of fewer than 50 cases of squamous cell carcinoma and of the various lymphomas in the scar tissue around the implant.
https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(22)00549-6/fulltext
.png)