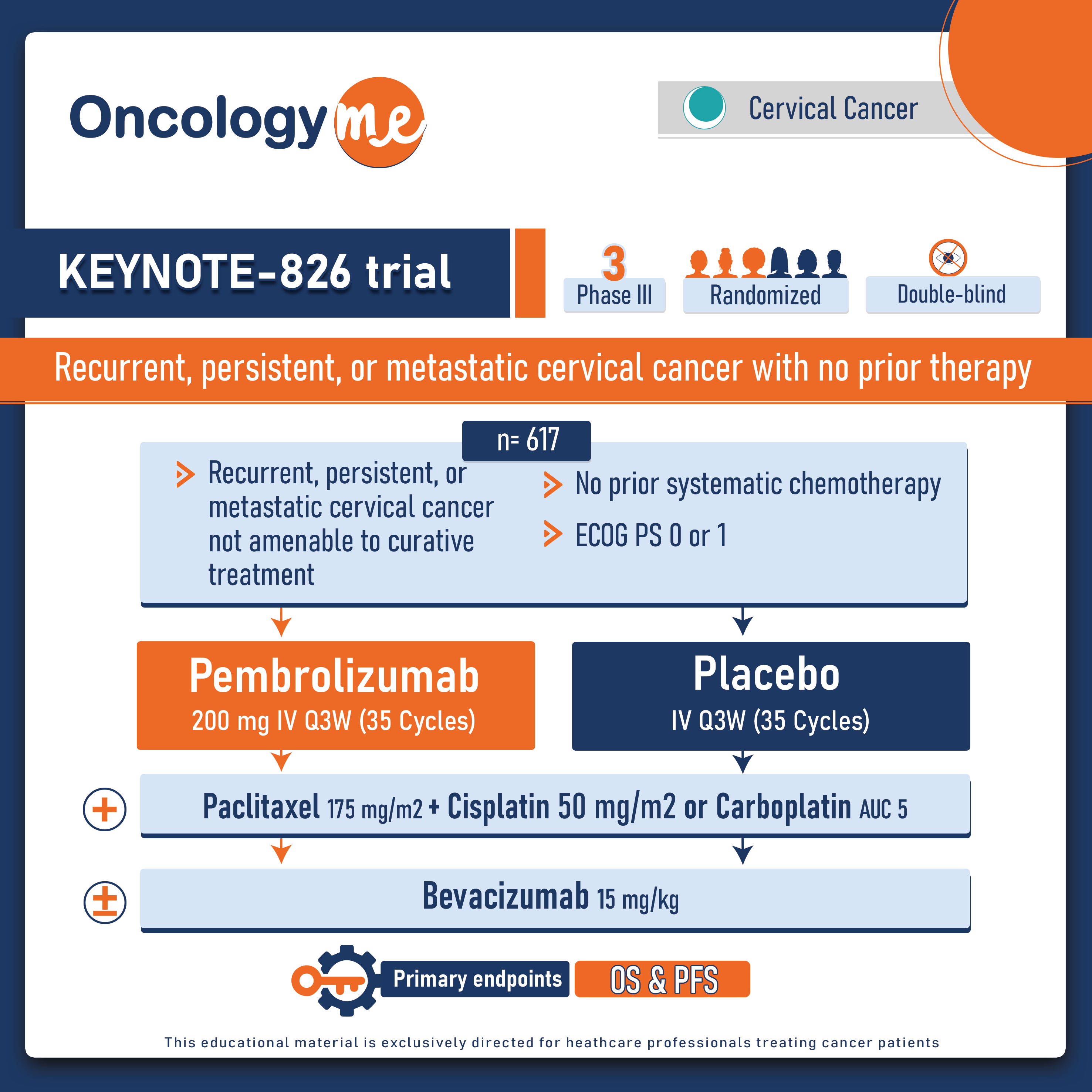
In the final analysis of the double-blind, phase III KEYNOTE-826 trial, The addition of #pembrolizumab to chemotherapy with or without #bevacizumab improved the clinical outcomes in patients with recurrent, persistent, or metastatic #cervical cancer with no prior therapy.
In this randomized trial, 617 patients with with persistent, recurrent, or metastatic cervical cancer not previously treated with systemic chemo (prior radiosensitizing chemotherapy allowed) and not amenable to curative treatment (surgery or radiation) were randomized 1:1 to pembrolizumab 200 mg or placebo Q3W for up to 35 cycles + chemo (paclitaxel 175 mg/m2 + cisplatin 50 mg/m2 or carboplatin AUC 5), ± bevacizumab 15 mg/kg. Pts were stratified by their metastatic status at diagnosis, bevacizumab use, and PD-L1 CPS score (<1, 1 to <10, or ≥10). Dual primary end points were OS and PFS tested sequentially in the PD-L1 CPS ≥1, all-comer, and CPS ≥10 populations.
After median follow-up of more than 3 years (39.1 months). The addition of pembrolizumab to chemotherapy ± bevacizumab significantly reduced the risk of death by 40% in the PD-L1 CPS ≥1 population, by 37% in the all-comer population, and by 42% in the CPS ≥10 population. Median OS was 28.6m in the pembrolizumab + CTx arm Vs 16.5m in the placebo + CTx arm (HR= 0.6, P< 0.0001) in pts with PD-L1 CPS ≥ 1. In all-comer, the mOS was 26.4m vs 16.8m in both arms respectively (HR=0.63, P<0.0001). Moreover, in pts with PD-L1 CPS≥ 10, mOS was 29.6m vs 17.4m in both arms respectively (HR=0.58, P <0.0001).
Similarly, PFS outcomes were also superior with the addition of pembrolizumab to chemotherapy in patients with PD-L1 CPS ≥ 1 (H=0.58; P <0.0001), in the overall population (HR=0.61; P <0.0001), and in patients with PD-L1 CPS ≥ 10 (HR=0.52; P <0.0001).
The pembro + chemo benefit was evident regardless of bev use. Grade ≥3 AE incidence was 82.4% in the pembro + chemo arm and 75.4% in the placebo + chemo arm. The most common grade ≥3 AEs were anemia (30.3% vs 27.8%), neutropenia (12.4% vs 9.7%), and hypertension (10.4% vs 11.7%).
Continuing research aims to evaluate how pembrolizumab and other immune checkpoint inhibitors can be utilized at earlier stages of the disease. While the phase 3 CALLA trial revealed no significant improvement in effectiveness when the durvalumab was added to chemoradiation for patients with locally advanced cervical cancer, there are other trials still in progress. Currently, the randomized phase III KEYNOTE-A18 trial is assessing the potential benefits of adding pembrolizumab to chemoradiotherapy for patients with locally advanced cervical cancer."
.png)