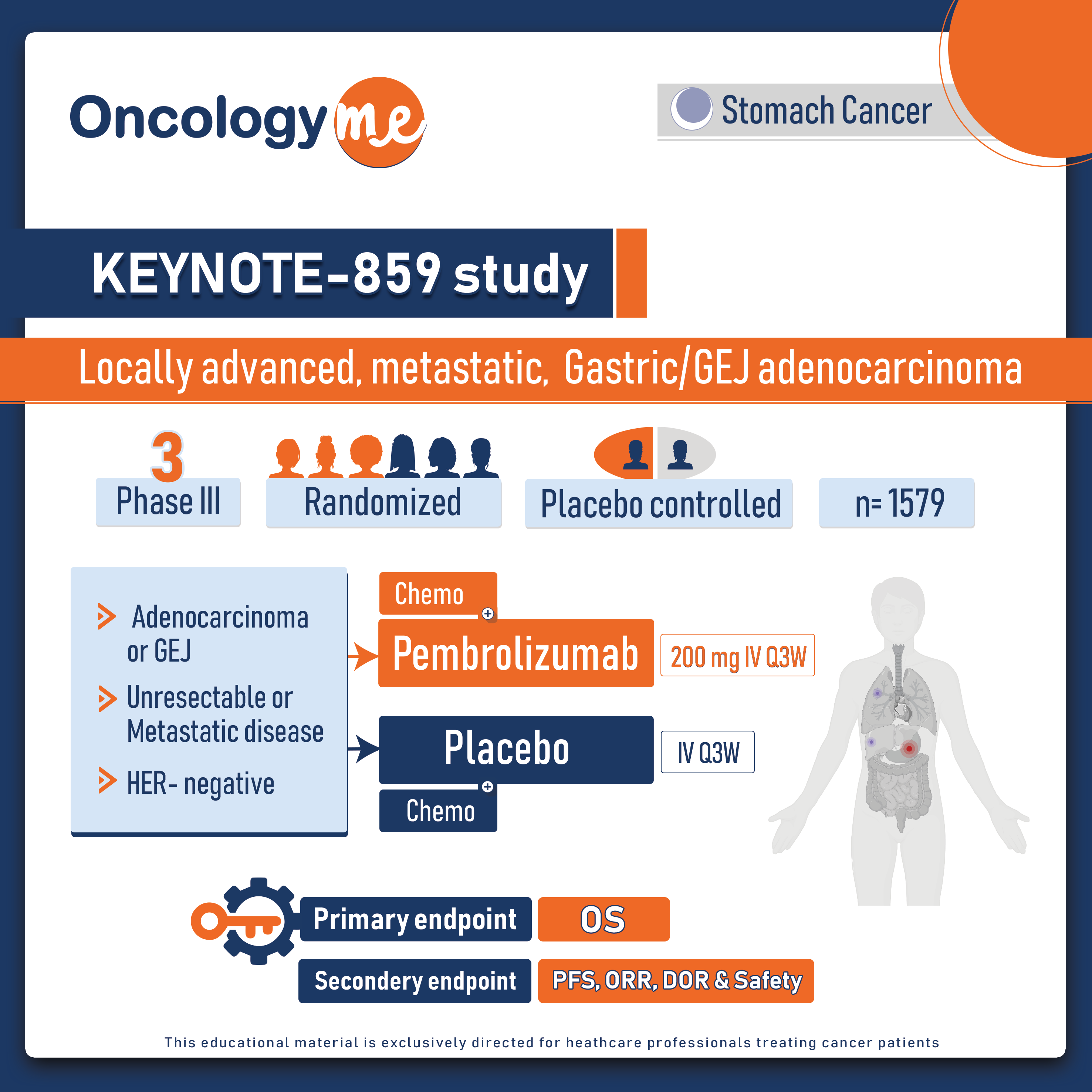
In the recent update of KEYNOTE-859 study, The addition of #pembrolizumab to chemotherapy significantly improved OS, PFS, and ORR in the PD-L1 CPS ≥1 and ≥10 populations in patients with advanced HER2-negative #gastric or #gastroesophageal junction (G/GEJ) cancer.
In this randomized trial, 1579 eligible patients with HER2-negative, previously untreated locally advanced or metastatic G/GEJ adenocarcinoma and known PD-L1 CPS were randomized 1:1 to pembrolizumab (pembro) 200 mg or placebo IV Q3W for ≤35 cycles, both given with either of 5-FU + cisplatin (FP) or capecitabine + oxaliplatin (CAPOX). the primary endpoint of OS, secondary endpoints included PFS and ORR which were also tested in the PD-L1 CPS ≥1 and ≥10 populations.
In the ITT population of the KEYNOTE-859 study, pembro + chemotherapy (chemo) significantly improved OS (HR 0.78, P < 0.0001), PFS (HR 0.76, P < 0.0001), and ORR (51.3% vs 42.0%; P = 0.00009) vs placebo + chemo at the protocol-specified interim analysis.
In the protocol-specified PD-L1 combined positive score (CPS) ≥1, median OS was 13.0 m for pembro + chemo vs 11.4 m for placebo + chemo (HR 0.74, P < 0.0001), median PFS was 6.9 m vs 5.6 m (HR 0.72, P < 0.0001), ORR was 52.1% vs 42.6% (P = 0.00041), and median DOR was 8.3 m vs 5.6 m.
In the PD-L1 CPS ≥10 population, median OS was 15.7 m with pembro + chemo vs 11.8 m with placebo + chemo (HR 0.65, P < 0.0001), median PFS was 8.1 m vs 5.6 m (HR 0.62, P < 0.0001), ORR was 60.6% vs 43.0% (P = 0.00002), and median DOR was 10.9 m vs 5.8 m.
The safety profile of pembro + chemo was as expected. immune-mediated AE incidence was 27.1% vs 9.3% in the ITT population.
These data support pembro + chemo as a new first-line treatment option for pts with locally advanced or metastatic HER2-negative G/GEJ adenocarcinoma, regardless of PD-L1 expression.
.png)