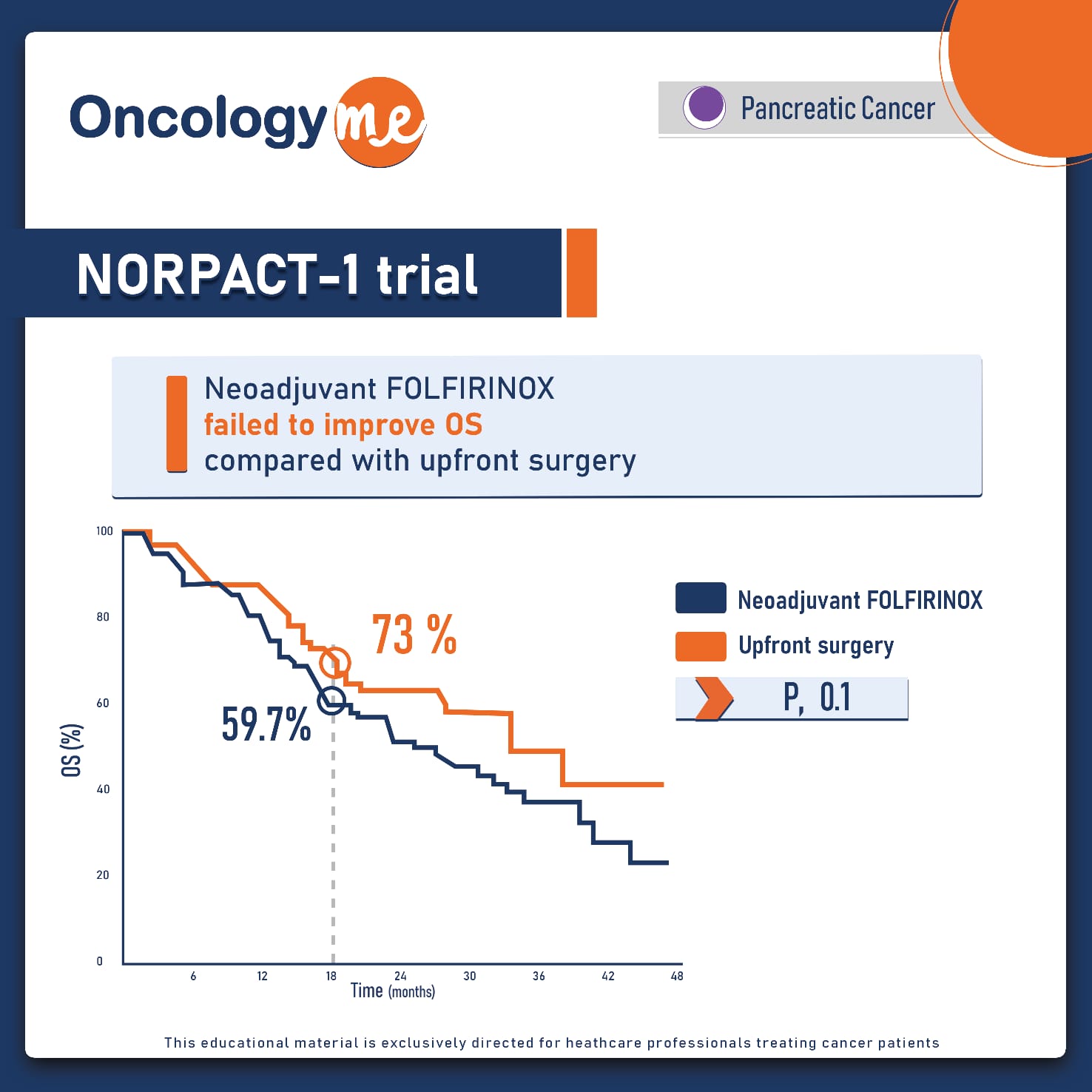
In the phase-II trial NORPACT-1 trial, Neoadjuvant #FOLFIRINOX failed to improve OS compared with upfront surgery in resectable #pancreatic head cancer.
In this randomized trial, that included 140 patients from 12 Nordic centers, Patients with resectable pancreatic head cancer were randomly assigned to receive either 4 cycles of neoadjuvant FOLFIRINOX followed by surgery and 8 cycles adjuvant #mFOLFIRINOX postoperatively, or upfront surgery followed by adjuvant mFOLFIRINOX (12 cycles). The primary endpoint was overall survival at 18 months after date of randomization. Median OS by ITT was 25.1 months with neoadjuvant chemotherapy and 38.5 months with upfront surgery (HR=-1.52, p=0.096). The proportion of patients alive at 18 months was 59.7 % and 73.0 %, respectively (p=0.100). Resection rates were 81.8% in the neoadjuvant group and 88.9% in the upfront surgery group (p=0.342). In the neoadjuvant group 61 (79.2%) patients initiated neoadjuvant FOLFIRINOX. Completion of the 4 planned cycles was only 60%, with dose reductions and delays in 70% and 28.3%.
These present data do not support the use of neoadjuvant chemotherapy as standard of care for patients with respectable pancreatic head cancer. Currently, 2 other RCTs compare perioperative with adjuvant administration of mFOLFIRINOX in patients with resectable pancreatic cancer, the Dutch PREOPANC-3 trial and the ALLIANCE A021806 trial from the US, both randomize eligible patients to either 8 cycles of neoadjuvant mFOLFIRINOX followed by surgery and 4 cycles of adjuvant mFOLFIRINOX or to surgery and 12 cycles of adjuvant mFOLFIRINOX. "
.png)