In the randomized, double-blind, phase III Keynote-756 study presented at ESMO2023 by Dr. Fatima Cardoso, the combination of #pembrolizumab and chemotherapy demonstrated a significant increase in the rate of pathological complete response (PCR) in patients with early-stage high-risk ER+/HER2- #breast_cancer compared to chemotherapy alone.
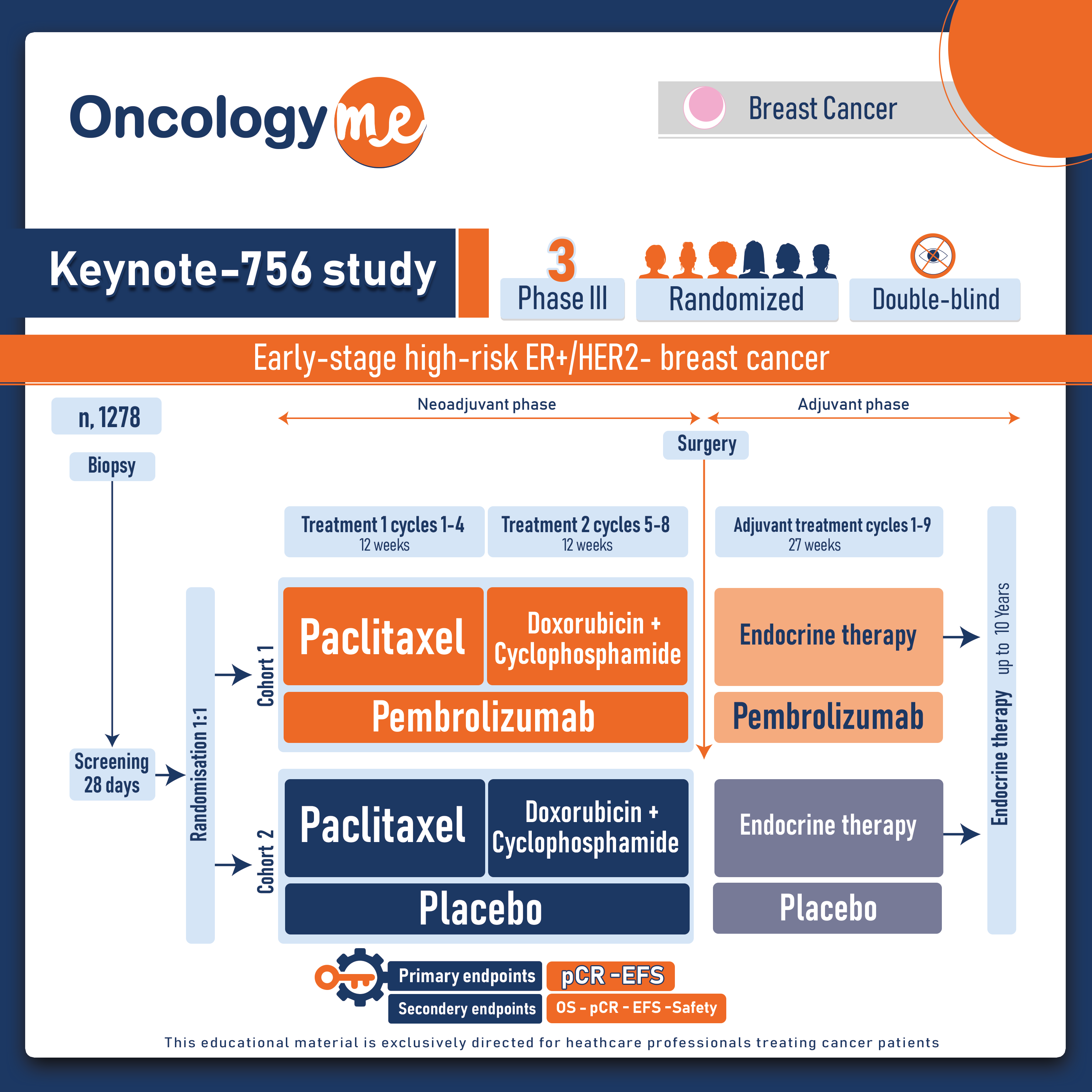
In this study, 1278 eligible patients with T1c-2 (≥2 cm) cN1-2 or T3-4 cN0-2, grade 3, invasive ductal ER+/HER2– breast cancer were randomly assigned in a 1:1 ratio to receive neoadjuvant pembrolizumab 200 mg Q3W or placebo, both given with weekly paclitaxel for 12 wk, then 4 cycles of doxorubicin or epirubicin + cyclophosphamide (neoadjuvant treatment). After definitive surgery (with or without radiation therapy), patients received pembrolizumab or placebo for 9 cycles along with standard endocrine therapy. Dual primary endpoints were pCR (ypT0/Tis ypN0) and event-free survival (EFS) while secondary endpoints included overall survival (OS), pCR defined as ypT0 ypN0 and ypT0/Tis, overall survival (OS); pCR, EFS, and OS in the PD-L1 population with a CPS > 1; and safety.
After median follow-up of 33.2 months, the addition of pembrolizumab to chemotherapy led to a significant improvement in pCR (ypT0/Tis ypN0) vs placebo + chemotherapy In the ITT population (24.3% vs 15.6%) with a difference of 8.5 percentage points; P=0.00005. Results were consistent for the secondary pCR definitions as well, ypT0 ypN0 (21.3% vs 12.8%) and ypT0/Tis (29.4% vs 18.2%). The benefit of adding pembrolizumab to chemotherapy on PCR was generally consistent across pre-specified subgroups.
During the neoadjuvant phase of the trial, the rates of grade ≥3 treatment-related adverse events (AE) were 52.5% with pembrolizumab + chemotherapy and 46.4% with placebo + chemotherapy, with 1 death in the pembrolizumab arm due to acute myocardial infarction. The most common TRAEs in the pembrolizumab and placebo arms were alopecia, nausea, anemia, fatigue.
In the neoadjuvant phase of the trial, 32.8% and 7.0% of patients in the pembrolizumab and placebo arms, respectively, experienced any-grade immune-mediated AEs. In the pembrolizumab arm, grade 3 to 5 and serious immune-mediated AEs occurred in 7.1% and 6.2% of patients in the pembrolizumab arm compared with 1.2% and 1.7% of those in the placebo arm. Immune-mediated AEs led to discontinuation of any drug in 7.7% and 1.6% of patients in the pembrolizumab and placebo arms, respectively.
The most common immune-mediated AEs in the pembrolizumab and placebo arms, respectively, were hypothyroidism (17.5% vs 1.7%), hyperthyroidism (9.0% vs 0.5%), pneumonitis (2.8% vs 1.4%), adrenal insufficiency (2.5% vs 0%), severe skin reactions (2.2% vs 0.5%), hypophysitis (1.9% vs 0.2%), thyroiditis (1.7% vs 0.3%), hepatitis (1.3% vs 0.5%), colitis (0.9% vs 0.8%), and vasculitis (0.8% vs 0.6%).
EFS results are still immature and continue to be evaluated Moreover, the increased immune toxicity associated with pembrolizumab remains a critical concern.
.png)