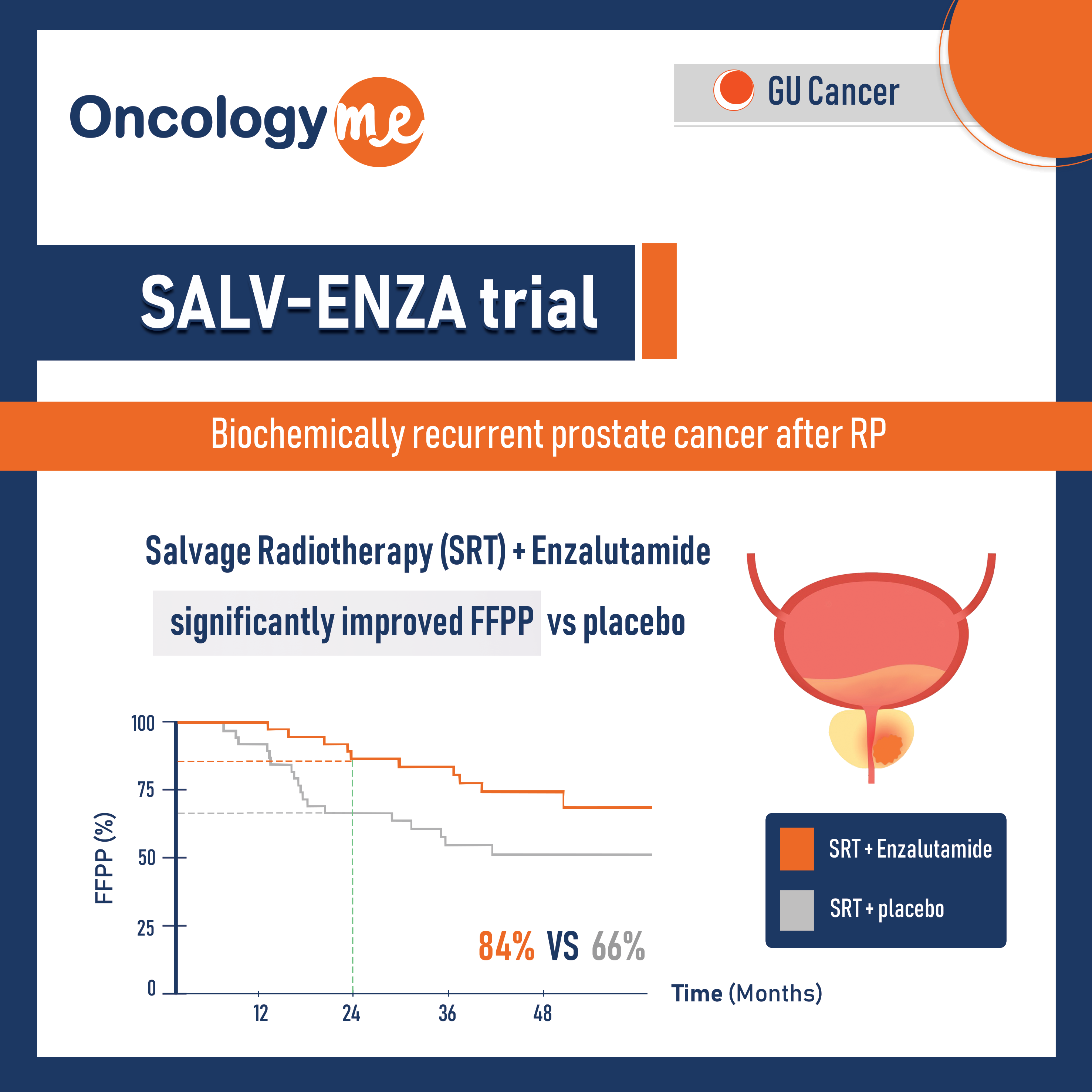
In the multicenter, phase II SALV-ENZA trial, 86 men with biochemically recurrent #prostate_cancer after RP were randomized to either salvage Radiotherapy plus #enzalutamide 160 mg once daily or matching placebo for 6 months. After 2 months of study drug therapy, external-beam radiation (66.6‐70.2 Gy) was administered to the prostate bed (no pelvic nodes).
After median follow-up of 34 months, Freedom From PSA Progression (FFPP) was significantly improved with enzalutamide versus placebo (HR, 0.42; P = .031), and 2-year FFPP was 84% versus 66%, respectively. Subgroup analyses demonstrated differential benefit of ENZA in men with pT3 (HR, 0.22) versus pT2 disease (HR, 1.54); P interaction = .019 and R1 (HR, 0.14) versus R0 disease (HR, 1:00); p interaction = .023).
.png)