In the phase 2 SERENA 2 trial, presented at the SABCS, treatment with the oral #SERD, #camizestrant monotherapy, resulted in a more than doubling of PFS vs standard-of-care #fulvestrant in postmenopausal women with ER–positive, HER2-negative advanced #breast_cancer.
In this randomized, multidose trial, metastatic breast cancer patients with recurrence/progression on ≥1 line of ET; no more than 1 line of ET or CT in advanced settings, were randomized to camizestrant in 2 different doses or fulvestrant.
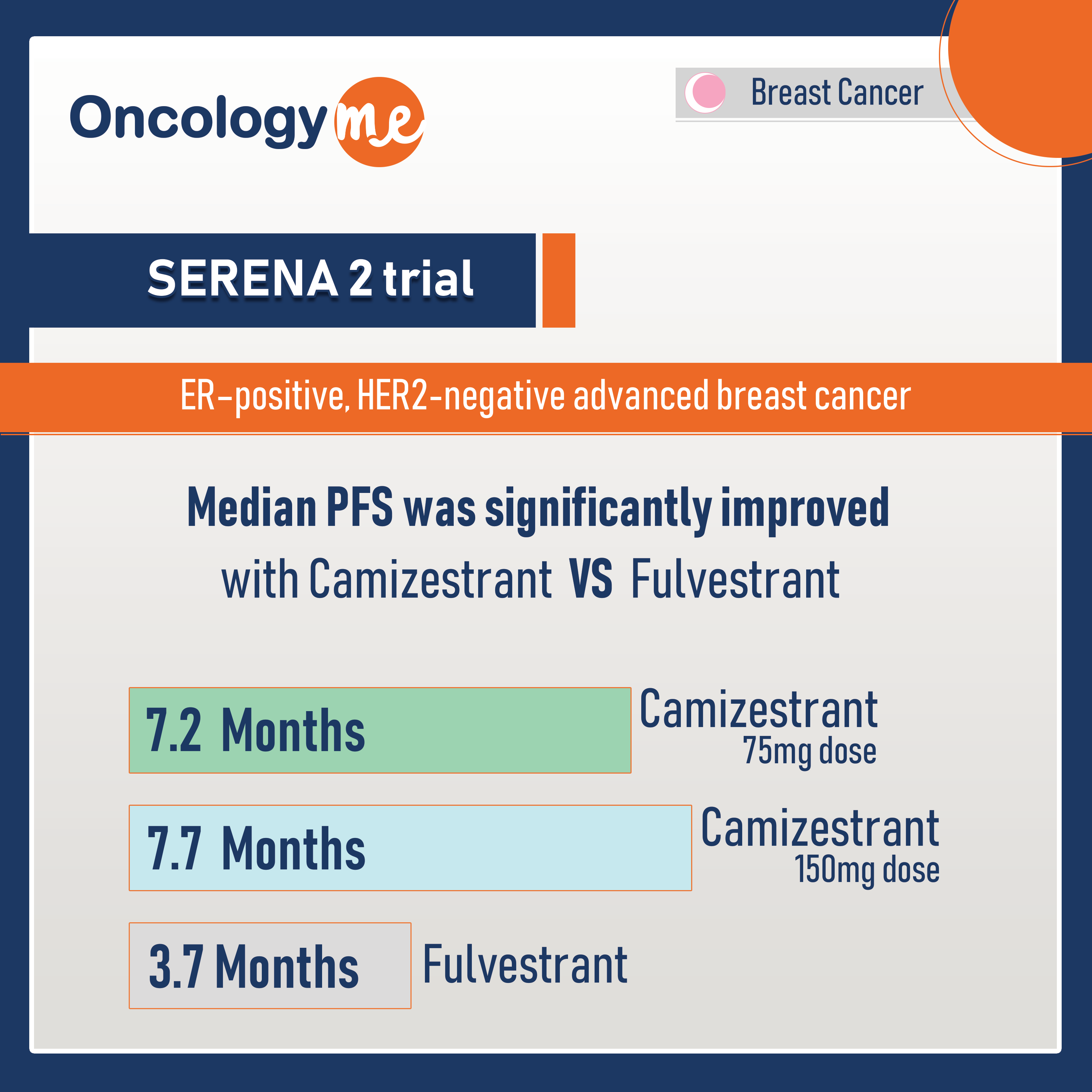
Median PFS, which was the primary end point, was significantly improved with camizestrant, 7.2 months for 75mg dose and 7.7 months for 150mg dose versus 3.7 months for fulvestrant (HR= 0.58 and 0.67 respectively).
.png)