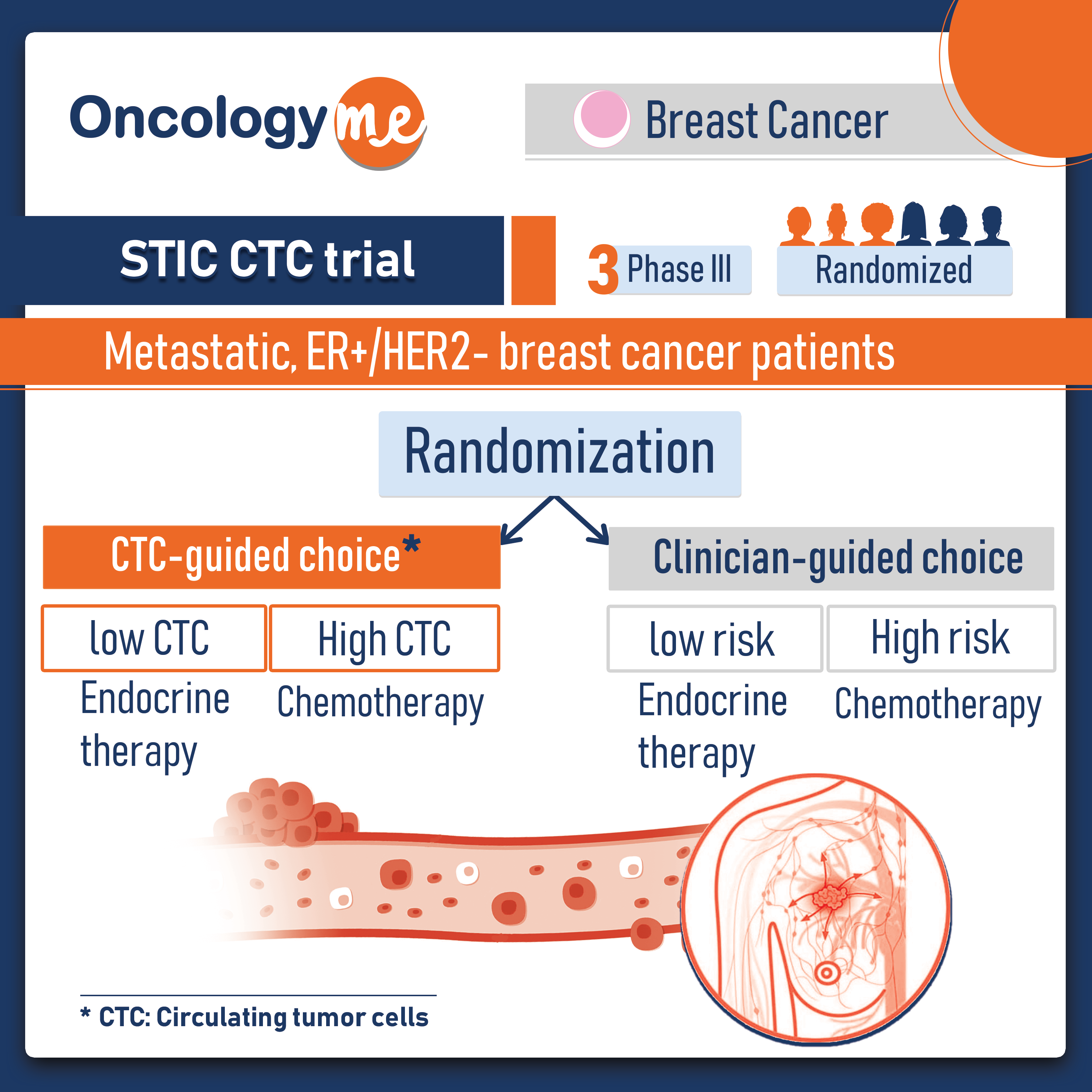
In the phase 3 STIC CTC trial, overall survival (OS) was improved when circulating tumor cell (CTC) count was used to guide the first-line treatment strategy vs physician’s choice in patients with hormone receptor–positive/HER2-negative metastatic breast cancer.
In this trial, 755 women with metastatic, ER+/HER2- breast cancer were randomized to either CTC-driven choice arm, or the clinician-driven choice arm. In the CTC-driven choice arm, patients with a low CTC count received endocrine therapy and those with a high CTC count received chemotherapy while in the clinician-driven choice arm, patients with a clinically low risk disease received endocrine therapy while those with a clinically high risk received chemotherapy.
In patients with clinically low risk/CTC high group, the median OS was 51.8 months in patients treated with chemotherapy compared with 35.4 months in those treated with endocrine therapy (HR, 0.53; P = .001). It should be noted that CDK4/6 inhibitors, which are considered the modern standard, were not included in the treatment choices.
.png)