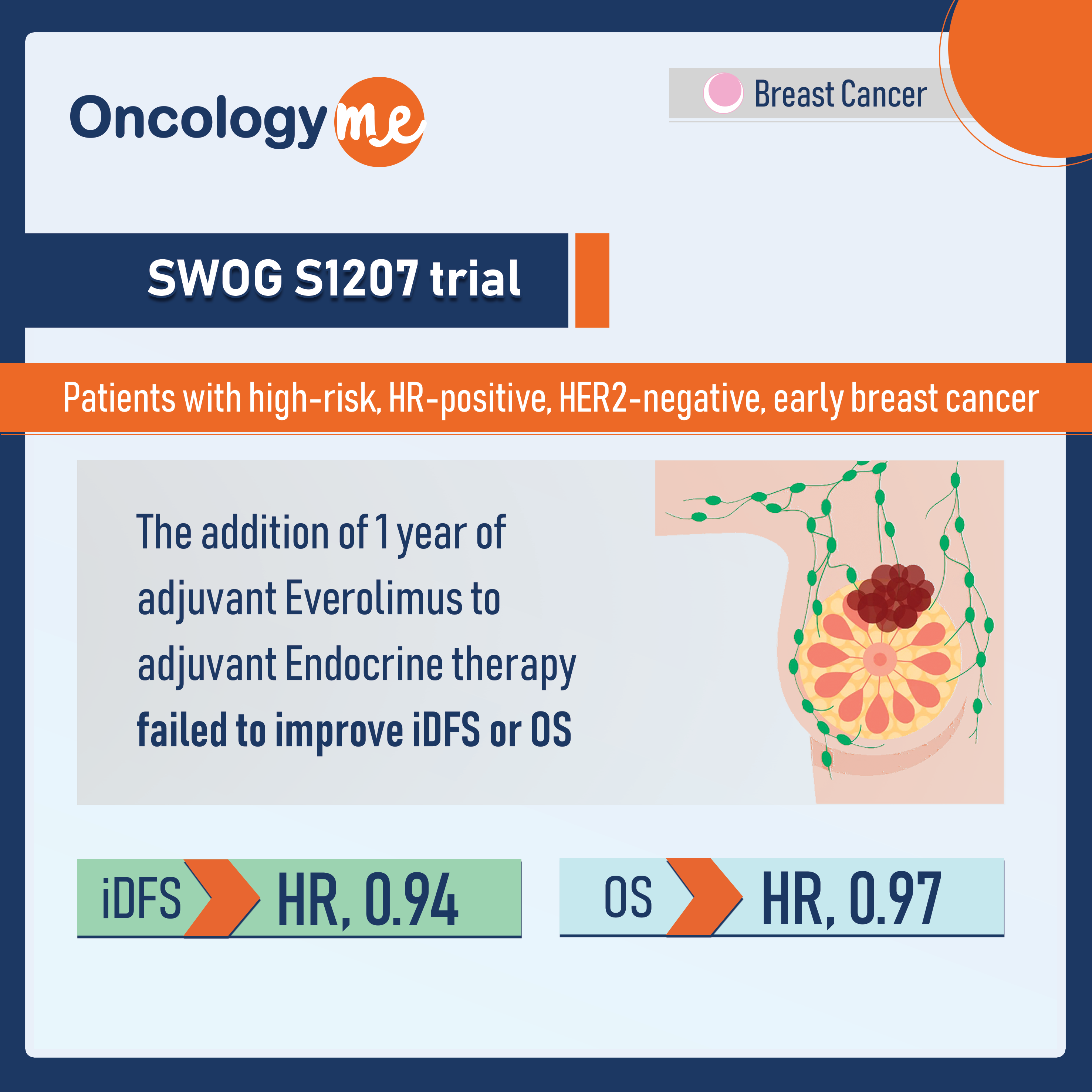
In the phase 3 SWOG S1207 trial, the addition of 1 year of adjuvant Everolimus to adjuvant endocrine therapy failed to improve iDFS or OS in patients with high-risk, HR-positive, HER2-negative, early breast cancer.
In the study that was presented by in SABCS-2022, eligible patients were randomized to physician’s choice of adjuvant endocrine therapy plus 1 year of Everolimus (n = 971), or endocrine therapy plus placebo (n = 968). The primary endpoint was iDFS.
After a median follow-up of 55.2 months, iDFS wasn’t improved with the addition of everolimus (HR, 0.94; P= .52). Similarly, no OS difference was observed (HR, 0.97; P = .84). In a subgroup analysis, premenopausal patients seemed to achieve better iDFS with everolimus (HR, 0.64; P = .02).
.png)