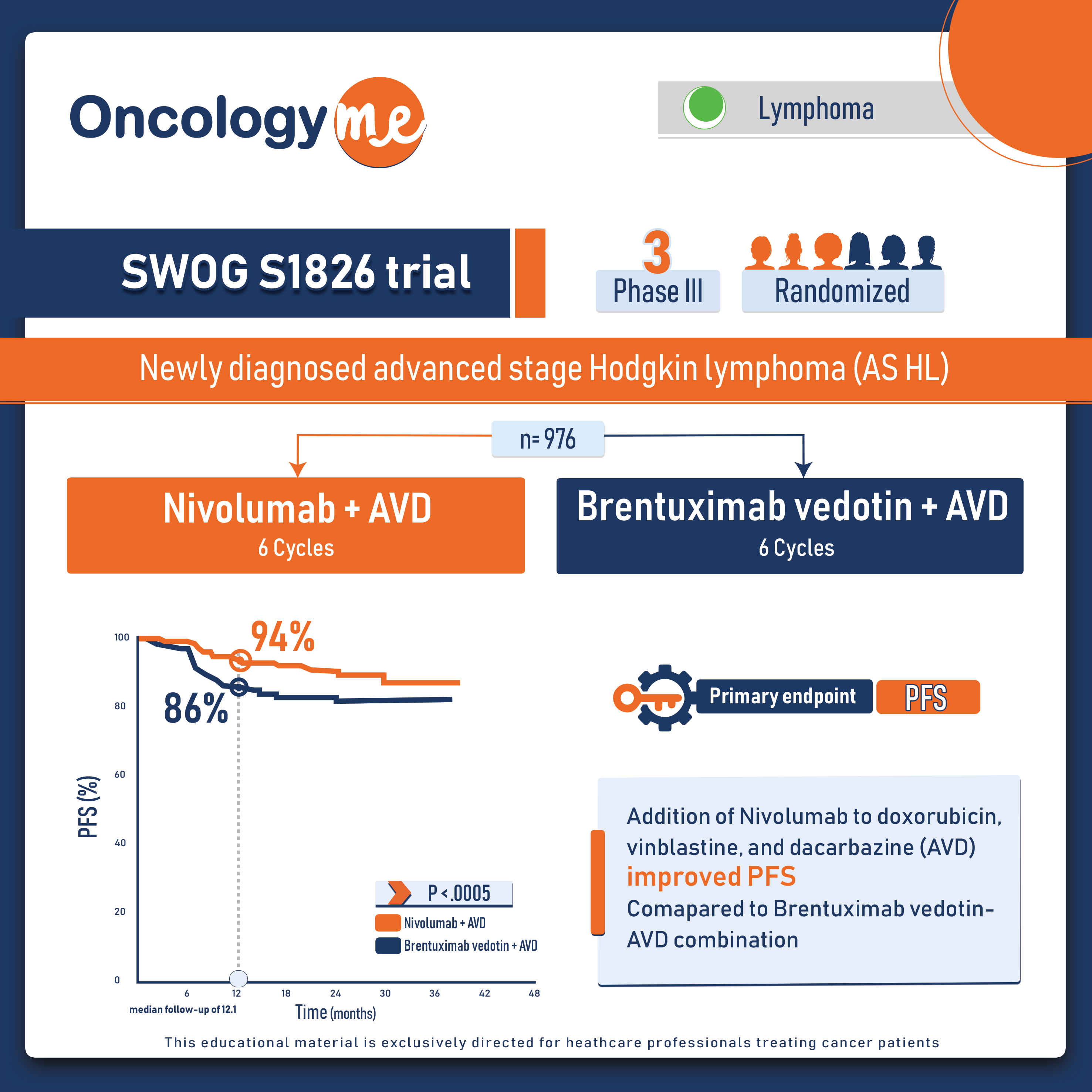
In the phase III SWOG S1826 trial, the addition of #nivolumab to doxorubicin, vinblastine, and dacarbazine (AVD) proved to be superior to #Brentuximab vedotin-AVD combination as regard progression-free survival in patients with newly diagnosed advanced stage #Hodgkin lymphoma (AS HL).
In this randomized trial conducted by the adult and pediatric cooperative groups of the National Clinical Trials Network (NCTN),976 eligible patients aged 12 years or more were randomized 1:1 to either 6 cycles of N-AVD or BV-AVD. Patients who received BV-AVD treatment were mandated to also receive GCSF, whereas it was optional for those on N-AVD. Patients were allowed to receive radiation therapy (RT) for residual metabolically active lesions seen on the final PET scan. The primary endpoint was PFS; secondary endpoints included OS, event-free survival, patient-reported outcomes (PROs), and safety.
At the second planned interim analysis, at a median follow-up of 12.1 months, the study met its primary endpoint. 1-year PFS was 94% with N-AVD arm and 86% with BV-AVD with a significant HR of 0.48, (P < .0005). This represents a 52% reduction in the risk for disease progression or death with N-AVD. Although overall survival is still immature, there’s a trend in favor of the nivolumab-AVD combination therapy at this early point. 11 deaths (7 due to adverse events, AE) were observed after BV-AVD compared to 4 after N-AVD (3 due to AE).
The rate of grade 3 or higher adverse events was higher with nivolumab than with brentuximab vedotin: 48.4% vs 30.5%. The rate of grade (gr) ≥ 3 hematologic AE was 48.4% (45.1% gr ≥ 3 neutropenia) after N-AVD compared to 30.5% (23.9% gr ≥ 3 neutropenia) after BV-AVD. Rates (any gr) of febrile neutropenia (5.6% N vs 6.4% BV), pneumonitis (2.0% N vs 3.2% BV), ALT elevation (30.7% N vs 39.8% BV), and colitis (1% N vs 1.3% BV) were similar. Hypo/hyperthyroidism was more frequent after N-AVD (7%/3% N vs <1% BV) while peripheral neuropathy (any gr) was more common after BV-AVD (sensory: 28.1% N vs 54.2% BV; motor: 4% N vs 6.8% BV).
This trial is yet to be the biggest study on Hodgkin’s lymphoma (HL) in the history of the National Clinical Trials Network (NCTN), which is a significant milestone towards harmonizing the treatment landscape of both pediatric and adult patients with HL. These results have strong potential to change the standard of care for previously untreated patients with advanced-stage Hodgkin lymphoma. "
.png)