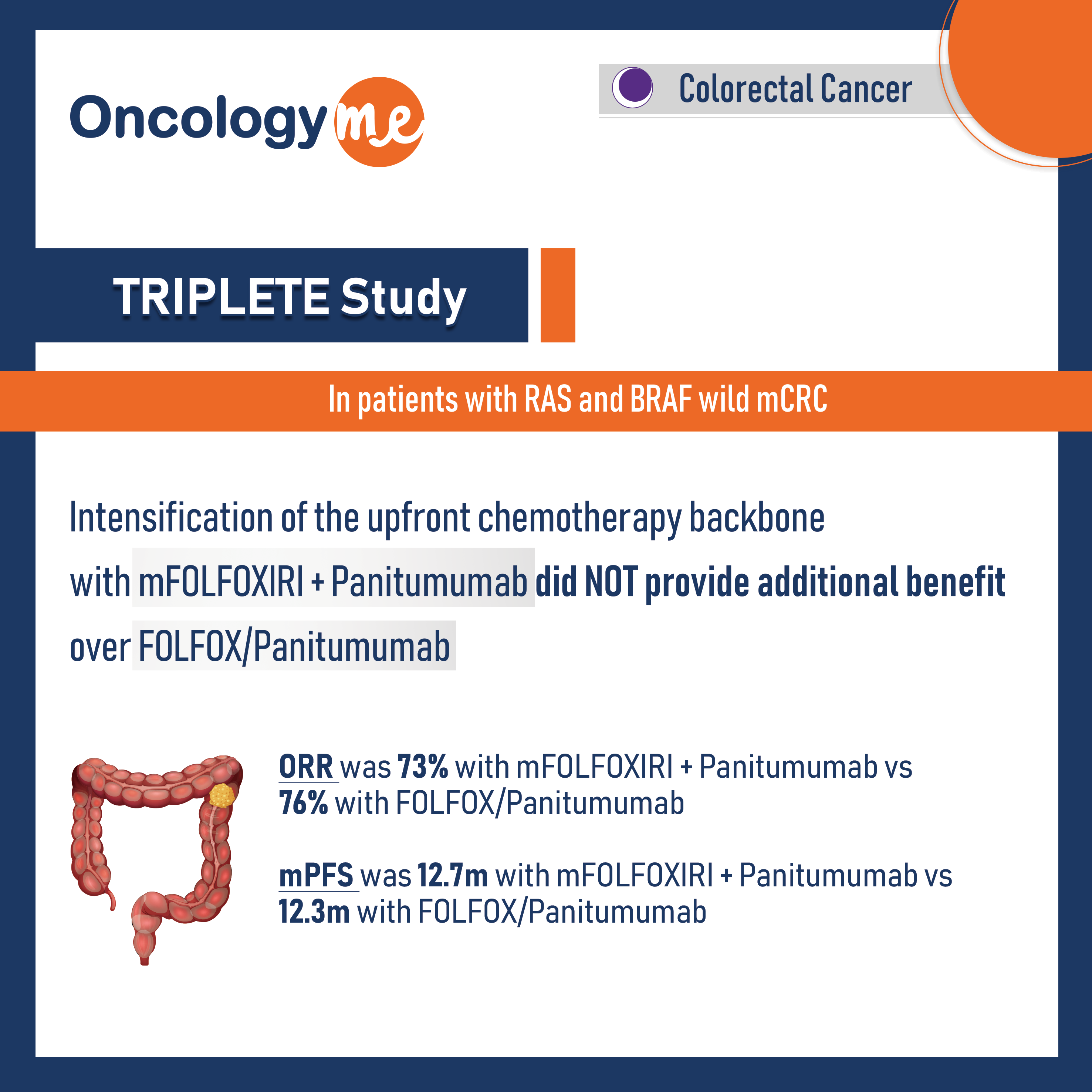
In phase III TRIPLETE Study by GONO group, the intensification of the upfront chemotherapy backbone in combination with #panitumumab did not provide additional benefit in patients with RAS and BRAF wild #mCRC.
Eligible patients (n=435) were randomly assigned 1:1 to modified FOLFOX/panitumumab (control group) or mFOLFOXIRI/panitumumab (experimental group) up to 12 cycles, followed by fluorouracil/-leucovorin/panitumumab until disease progression. The primary end point, ORR was 73% in the experimental group versus 76% in the control group (OR: 0.87; P= 0.526). Additionally, mPFS was 12.7 months in the experimental group and 12.3 months in the control group (HR, 0.88; P= 0.277)
.png)