News
EMERALD trial
Oncologyme
/ Mar 26, 2023
.png)
In a study published in the Journal of clinical oncology, Oral #SERD, #Elacestrant significantly improved PFS compared to standard of...
Cemiplimab FDA approval in NSCLC
Oncologyme
/ Mar 26, 2023
.png)
On November 8, 2022, the FDA approved cemiplimab-rwlc in combination with platinum-based chemotherapy as first line therapy for patients with...
NEOPAN trial
Oncologyme
/ Mar 26, 2023

In phase III NEOPAN study presented by Dr. Ducreux, FOLFIRINOX improved progression-free survival (PFS) compared to gemcitabine in #locally_advanced unresectable...
Surgery omission in early breast cancer
Oncologyme
/ Mar 26, 2023

In a multicentre, single-arm, phase 2 trial conducted by MD Anderson Cancer Center, carefully selected early stage #TNBC or #HER2...
COSMIC-312 trial
Oncologyme
/ Mar 26, 2023
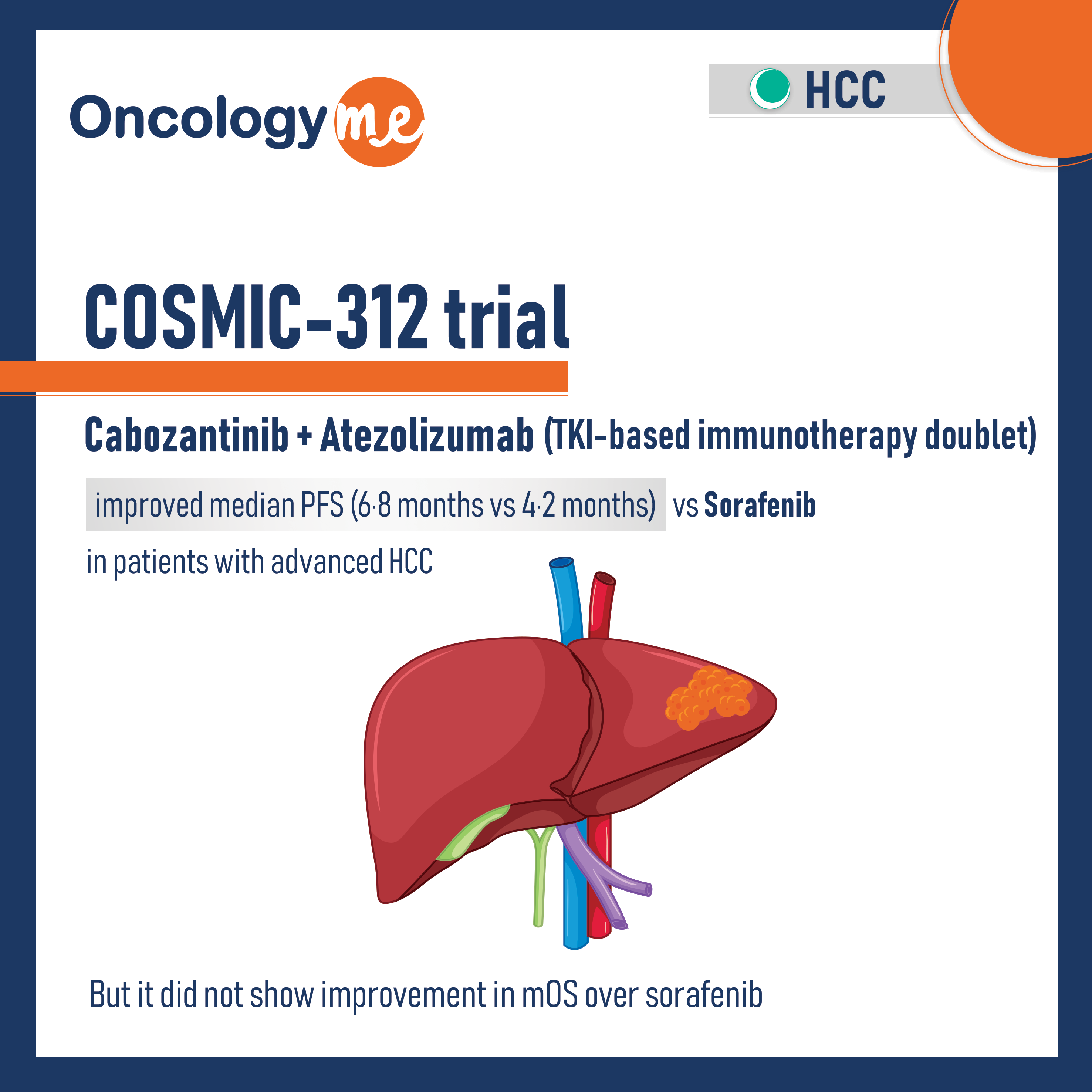
In COSMIC-312 trial, first line #Cabozantinib plus #atezolizumab improved PFS versus #sorafenib for patients with advanced HCC. In this phase...
FDA safety recommendation
Oncologyme
/ Mar 26, 2023
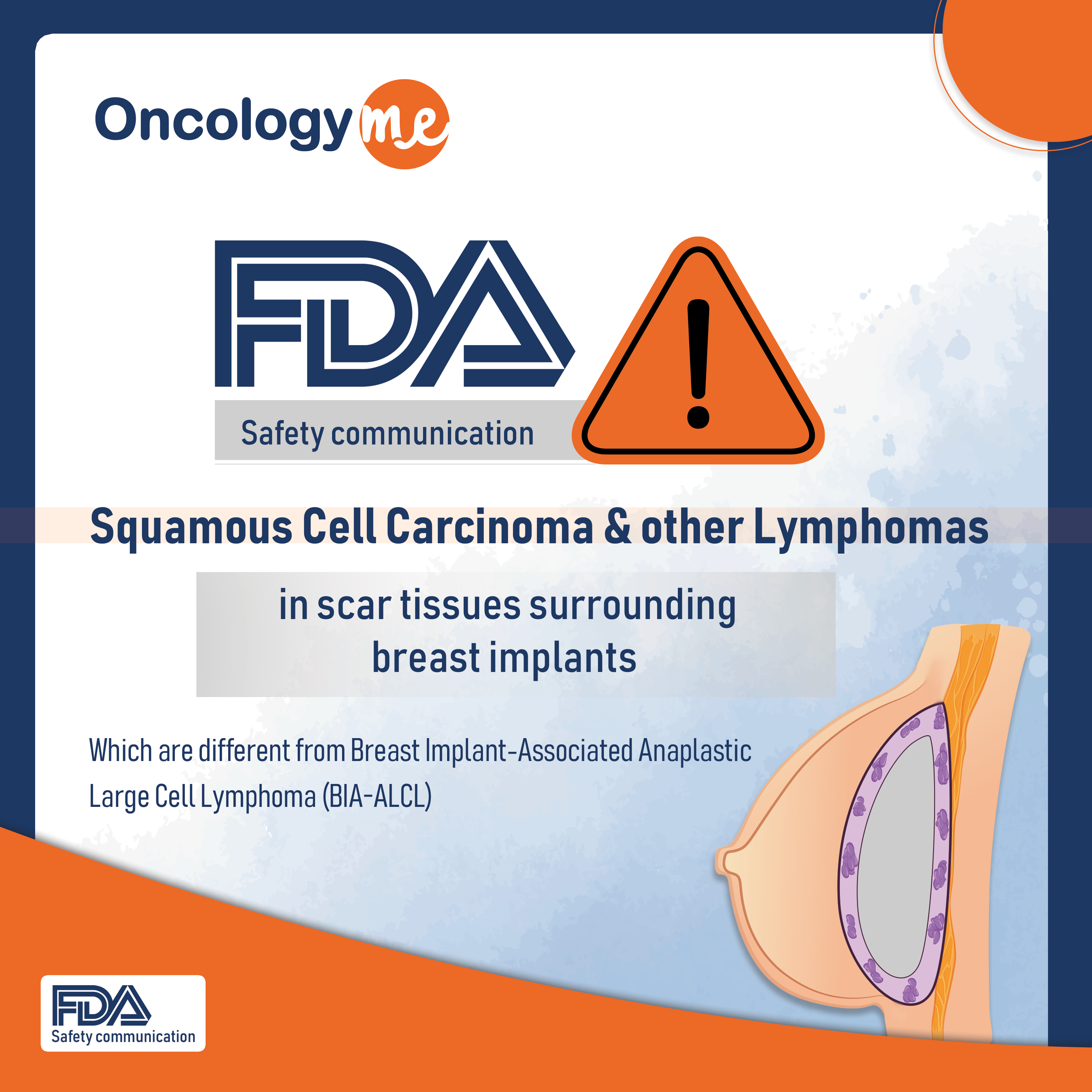
On Sept 8, 2022, the #FDA issued a safety communication to highlight reports of squamous cell carcinoma and various #lymphomas...
RTOG 1112 trial
Mar 26, 2023

The addition of stereotactic body radiation therapy (SBRT) to sorafenib for patients with hepatocellular carcinoma (HCC) improved PFS and OS...
POSEIDON trial
Mar 26, 2023
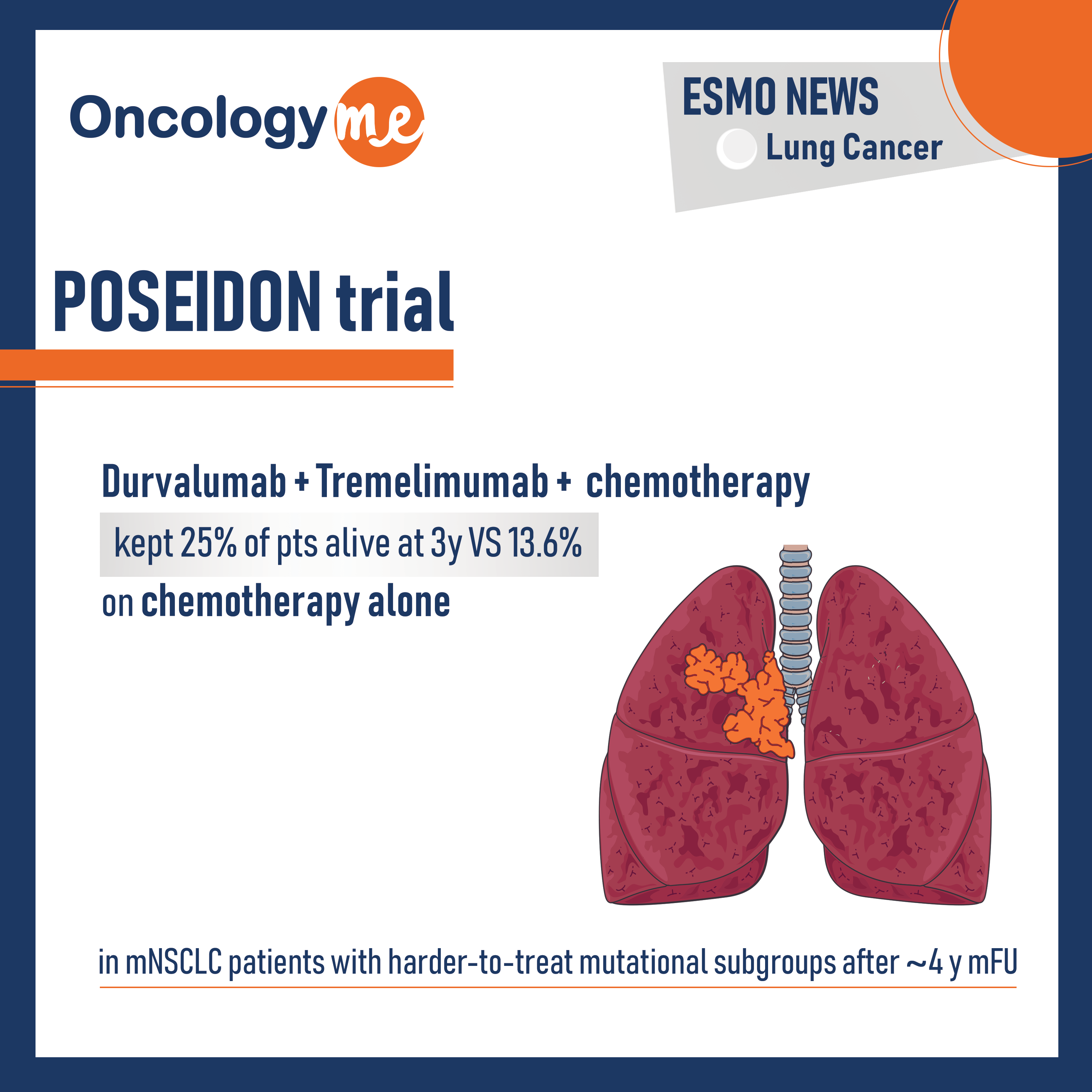
The results of the exploratory analysis from POSEIDON trial were presented by Dr. Melissa Johnson. In this phase III trial,...
PRESTO trial
Oncologyme
/ Mar 26, 2023

ADT Intensification might be the resort to delay biochemical progression in High risk biochemical relapse (BCR) #prostate cancer after Radical...
HIMALAYA trial
Oncologyme
/ Mar 26, 2023
.png)
The #FDA has approved Tremelimumab plus Durvalumab for patients with unresectable #Hepatocellular Carcinoma. This approval was based on the results...
.png)